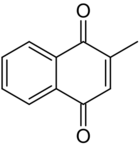
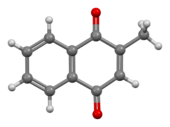
Chemistry:Menadione
This article is missing information about drugbox or more Chembox Pharmacology. (January 2021) |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylnaphthalene-1,4-dione | |
| Other names
Menaphthone; Vitamin K3; β-Methyl-1,4-naphthoquinone; 2-Methyl-1,4-naphthodione; 2-Methyl-1,4-naphthoquinone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H8O2 | |
| Molar mass | 172.183 g·mol−1 |
| Appearance | Bright yellow crystals |
| Density | 1.225g/cm3 |
| Melting point | 105 to 107 °C (221 to 225 °F; 378 to 380 K) |
| Insoluble | |
| Pharmacology | |
| 1=ATC code }} | B02BA02 (WHO) |
| |
| |
| Legal status |
|
| Hazards | |
| Flash point | 113.8 °C (236.8 °F; 386.9 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
0.5 g/kg (oral, mouse) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Menadione is a natural[3] organic compound with the formula C6H4(CO)2C2H(CH3). It is an analog of 1,4-naphthoquinone with a methyl group in the 2-position.[4] It is sometimes called vitamin K3. Use is allowed as a nutritional supplement in animal feed because of its vitamin K activity.
Biochemistry
Menadione is converted to vitamin K2 (specifically, MK-4) by the prenyltransferase action of vertebrate UBIAD1.[3] This reaction requires the hydroquinone (reduced) form of K3, menadiol, produced by NQO1.[5]
Menadione is also a circulating form of vitamin K, produced in small amounts (1–5%) after intestinal absorption of K1 and K2. This circulation explains the uneven tissue distribution of MK-4, especially since menadione can penetrate the blood–brain barrier. The cleavage enzyme is yet to be identified. As K3 is known to be toxic in large amounts, researchers speculate that the cleavage process is closely regulated.[5]
Terminology
The compound is variously known as vitamin K3[6] and provitamin K3.[7] Proponents of the latter name generally argue that the compound is not a real vitamin due to its artificial status (prior to its identification as a circulating intermediate) and its lack of a 3-methyl side chain preventing it from exert all the functions (specifically, it cannot act as a cofactor for GGCX in vitro)[8] of the K vitamins.
Uses
It is an intermediate in the chemical synthesis of vitamin K by first reduction to the diol menadiol, which is susceptible to coupling to the phytol.[9] It is a useful intermediate for organic synthesis in general, as it can be made and modified in a number of ways.[10]
Menadione can be used to generate reactive oxygen species to perform flow cytometry analysis on. It can also be used in microbiological evaluation to, for example, detect fastidious microorganisms.[11]
Animal feed
In the United States, menadione is used in various types of animal feed and is described as having a history of safe use for this purpose, being used in poultry feed prior to 1958.[12]
Low-dose menadione is used as an inexpensive micronutrient for livestock in many countries. Forms of menadione are also included in some pet foods in developed countries as a source of vitamin K. These doses have yielded no reported cases of toxicity from menadione in livestock or pets. Although handling may be hazardous, the European Food Safety Authority found in 2013 that it is an effective source of vitamin K in animal nutrition that does not pose a risk to the environment.[13]
Human use
Despite the fact that it can serve as a precursor to various types of vitamin K, menadione is generally not used as a nutritional supplement in economically developed countries. Menadione for human use at pharmaceutical strength is available in some countries with large lower income populations, such as India.[2] The typical daily dose is 10 mg oral or 2 mg parenteral.[14] It is used in the treatment of hypoprothrombinemia outside of the United States.[2]
Toxicology
Menadione is not believed to be carcinogenic.[15] K3 can cause generation of reactive oxygen species (ROS) by redox cycling and arylation of thiols using its reactive 3-position.[5] ROS generation explains various toxic effects of excessive menadione, including DNA damage and cell death,[15] or on a whole-animal level, cardiac and renal toxicity in rats.[16]
References
- ↑ The Merck Index, 11th Edition, 5714
- ↑ 2.0 2.1 2.2 "Menadione drug information". https://www.drugsupdate.com/generic/view/1153/Menadione.
- ↑ 3.0 3.1 Hirota, Yoshihisa; Tsugawa, Naoko; Nakagawa, Kimie; Suhara, Yoshitomo; Tanaka, Kiyoshi; Uchino, Yuri; Takeuchi, Atsuko; Sawada, Natsumi et al. (2013-11-15). "Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats". The Journal of Biological Chemistry 288 (46): 33071–33080. doi:10.1074/jbc.M113.477356. ISSN 1083-351X. PMID 24085302.
- ↑ Fox, ed (2008). "Cytotoxicity mechanism of two naphthoquinones (menadione and plumbagin) in Saccharomyces cerevisiae". PLOS ONE 3 (12): e3999. doi:10.1371/journal.pone.0003999. PMID 19098979. Bibcode: 2008PLoSO...3.3999C.
- ↑ 5.0 5.1 5.2 Shearer, Martin J.; Newman, Paul (March 2014). "Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis". Journal of Lipid Research 55 (3): 345–362. doi:10.1194/jlr.R045559. ISSN 0022-2275. PMID 24489112.
- ↑ "Vitamin K3 (menadione)-induced oncosis associated with keratin 8 phosphorylation and histone H3 arylation". Molecular Pharmacology 68 (3): 606–15. September 2005. doi:10.1124/mol.105.013474. PMID 15939799.
- ↑ "Vitamin K" (in en). 2014-04-22. https://lpi.oregonstate.edu/mic/vitamins/vitamin-K.
- ↑ Buitenhuis, HC; Soute, BA; Vermeer, C (16 May 1990). "Comparison of the vitamins K1, K2 and K3 as cofactors for the hepatic vitamin K-dependent carboxylase.". Biochimica et Biophysica Acta (BBA) - General Subjects 1034 (2): 170–5. doi:10.1016/0304-4165(90)90072-5. PMID 2112953.
- ↑ Weber, Fritz; Rüttimann, August (2012). "Vitamin K". Ullmann's Encyclopedia Of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o27_o08.
- ↑ de Souza, AS; Ribeiro, RCB; Costa, DCS; Pauli, FP; Pinho, DR; de Moraes, MG; da Silva, FC; Forezi, LDSM et al. (2022). "Menadione: a platform and a target to valuable compounds synthesis.". Beilstein Journal of Organic Chemistry 18: 381–419. doi:10.3762/bjoc.18.43. PMID 35529893.
- ↑ "Menadione". https://www.sigmaaldrich.com/US/en/product/sigma/m5625.
- ↑ "Vitamin K Substances and Animal Feed". FDA. 2 April 2021. https://www.fda.gov/animal-veterinary/safe-feed/vitamin-k-substances-and-animal-feed.
- ↑ EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (January 2014). "Scientific Opinion on the safety and efficacy of vitamin K3 (menadione sodium bisulphite and menadione nicotinamide bisulphite) as a feed additive for all animal species.". EFSA Journal 12 (1): 3532. doi:10.2903/j.efsa.2014.3532.
- ↑ "Menadione (B02BA02)". https://www.whocc.no/atc_ddd_index/?code=B02BA02.
- ↑ 15.0 15.1 Hassan, Ghada S. (2013). "Menadione". Profiles of Drug Substances, Excipients and Related Methodology. 38. pp. 227–313. doi:10.1016/B978-0-12-407691-4.00006-X. ISBN 9780124076914.
- ↑ Chiou, TJ; Zhang, J; Ferrans, VJ; Tzeng, WF (31 December 1997). "Cardiac and renal toxicity of menadione in rat.". Toxicology 124 (3): 193–202. doi:10.1016/s0300-483x(97)00162-5. PMID 9482121.
External links
- Menadione in the Pesticide Properties DataBase (PPDB)
 |