Chemistry:Perfosfamide
 | |
| Clinical data | |
|---|---|
| Other names | Perfosfamide |
| Routes of administration | Extracorporal treatment of cellular masses; intravenously |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
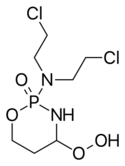
| Formula | C7H15Cl2N2O4P |
| Molar mass | 293.08 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Perfosfamide, or 4-hydroperoxycyclophosphamide (trade name Pergamid) was an experimental drug candidate for blood cancers that was rejected by the FDA in 1993 and never reached the market.
Intended use
Perfosfamide was used experimentally by oncologists in the 1980s for use in bone marrow transplant procedures for treatment of blood cancers. It was added to the tissue after it had been taken from the donor but before the tissue was administered to the patient to "purge" lymphocytes in order to prevent Graft Versus Host Disease, which results from donor lymphocytes attacking normal host cells and tissues, and to "purge" a patient's own (autologous) collected hematopoietic stem cells of any malignant cells it may still contain at the time of harvesting.[1][2]:255
In 1989 Nova Pharmaceutical took over development of the compound and in 1992, it submitted a new drug application to the FDA for using perfosamide to purge malignant cells in transplants for the treatment of acute myeloid leukemia. In the ensuing year, it merged with Scios to become Scios Nova. In March 1993, the FDA's Oncology Drugs Advisory Committee rejected the application because all the data was from the experimental uses, and no randomized clinical trial had been conducted.[3] At the review meeting, Scios Nova also announced that it would not fund further development of the drug candidate.[4]
Chemistry
Perfosfamide is an oxazaphosphorine compound, similar to mafosfamide and cyclophosphamide. Like those compounds it, is metabolized to 4-hydroxycyclophosphamide, which eventually gives rise to the two directly cytotoxic metabolites — phosphoramide mustard and acrolein.[5]
References
- ↑ "Aldehyde dehydrogenase-mediated cellular relative insensitivity to the oxazaphosphorines". Current Pharmaceutical Design 5 (8): 607–25. August 1999. doi:10.2174/1381612805666230110215319. PMID 10469894.
- ↑ "Chapter 20: Pharmacologic Purging of Bone Marrow.". Thomas' Hematopoietic Cell Transplantation. 457. John Wiley & Sons. 2004. ISBN 9781405112567.
- ↑ Fox, Jeffrey L. (April 1993). "Scios Nova may purge Pergamid after FDA setback.". Nature Biotechnology 11 (4): 439. doi:10.1038/nbt0493-439.
- ↑ "Scios Nova's Pergamid: No Further Trials Will Be Funded". The Pink Sheet. 8 March 1993. https://www.pharmamedtechbi.com/publications/the-pink-sheet/55/010/scios-novas-pergamid-no-further-trials-will-be-funded.
- ↑ "The chemistry of the metabolites of cyclophosphamide". Current Pharmaceutical Design 5 (8): 627–43. August 1999. doi:10.2174/1381612805666230110215458. PMID 10469895.
 |

