Chemistry:Ceftobiprole
 | |
| Clinical data | |
|---|---|
| Trade names | Zevtera |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
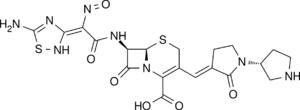
| Formula | C20H22N8O6S2 |
| Molar mass | 534.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ceftobiprole (Zevtera/Mabelio) is a fifth-generation[2] cephalosporin for the treatment of hospital-acquired pneumonia (excluding ventilator-associated pneumonia) and community-acquired pneumonia. It is marketed by Basilea Pharmaceutica in the United Kingdom, Germany, Switzerland and Austria under the trade name Zevtera, in France and Italy under the trade name Mabelio.[3] Like other cephalosporins, ceftobiprole exerts its antibacterial activity by binding to important penicillin-binding proteins and inhibiting their transpeptidase activity which is essential for the synthesis of bacterial cell walls. Ceftobiprole has high affinity for penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus strains and retains its activity against strains that express divergent mecA gene homologues (mecC or mecALGA251). Ceftobiprole also binds to penicillin-binding protein 2b in Streptococcus pneumoniae (penicillin-intermediate), to penicillin-binding protein 2x in Streptococcus pneumoniae (penicillin-resistant), and to penicillin-binding protein 5 in Enterococcus faecalis.[4]
Microbiology
Ceftobiprole has shown in vitro antimicrobial activity against a broad range of Gram-positive and Gram-negative pathogens. Among the Gram-positive pathogens, ceftobiprole has demonstrated good in vitro activity against methicillin-resistant Staphylococcus aureus, methicillin-susceptible Staphylococcus aureus and coagulase-negative staphylococci, as well as against strains of methicillin-resistant Staphylococcus aureus with reduced susceptibility to linezolid, daptomycin or vancomycin.[5] Ceftobiprole has also displayed potent activity against Streptococcus pneumoniae (including penicillin-sensitive, penicillin-resistant and ceftriaxone-resistant strains) and Enterococcus faecalis, but not against Enterococcus faecium. For Gram-negative pathogens, ceftobiprole has shown good in vitro activity against Haemophilus influenzae (including both ampicillin-susceptible and ampicillin-non-susceptible isolates), Pseudomonas aeruginosa and strains of Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis that do not produce extended-spectrum β-lactamases (ESBL). Like all other cephalosporins, ceftobiprole was inactive against strains that produce extended-spectrum β-lactamases.[6]
The efficacy of ceftobiprole has been demonstrated in two large randomized, double-blind, phase 3 clinical trials in patients with hospital-acquired and community-acquired pneumonia. Ceftobiprole was non-inferior to ceftazidime plus linezolid in the treatment of hospital-acquired pneumonia (excluding ventilator-acquired pneumonia) and non-inferior to ceftriaxone with or without linezolid in the treatment of community-acquired pneumonia.[7][8]
Pharmacology
Ceftobiprole is the active moiety of the prodrug ceftobiprole medocaril and is available for intravenous treatment only. The recommended dose is 500 mg as 2-hour infusion every 8 hours. It is mainly excreted renally. Dose adjustment is required for patients with moderate or severe renal impairment and for patients with end-stage renal disease, but no dose adjustment is needed by gender, ethnicity or age, in severely obese patients or in patients with hepatic impairment.[9]
Regulatory approvals
Ceftobiprole has been approved for the treatment of adult patients with hospital acquired pneumonia (excluding ventilator-acquired pneumonia) and community-acquired pneumonia in 12 European countries, Canada and Switzerland.[10]
Synonyms
References
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2015". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2015.
- ↑ "Ceftobiprole medocaril in the treatment of hospital-acquired pneumonia". Future Microbiology 10 (12): 1913–1928. 2015-01-01. doi:10.2217/fmb.15.115. PMID 26573022.
- ↑ "Basilea to launch Zevtera®/Mabelio® (ceftobiprole medocaril) in Europe through a commercial services provider". Basilea Pharmaceutica. http://www.basilea.com/News-and-Media/Basilea-to-launch-Zevtera-Mabelio-ceftobiprole-medocaril-in-Europe-through-a-commercial-services-provider/7dcfb10b-c4ef-5a82-8359-f88b1f36cb96/.
- ↑ "Ceftobiprole medocaril: a review of its use in patients with hospital- or community-acquired pneumonia". Drugs 74 (13): 1523–1542. September 2014. doi:10.1007/s40265-014-0273-x. PMID 25117196.
- ↑ "Ceftobiprole: a review of a broad-spectrum and anti-MRSA cephalosporin". American Journal of Clinical Dermatology 9 (4): 245–254. 2008. doi:10.2165/00128071-200809040-00004. PMID 18572975.
- ↑ "Ceftobiprole activity against over 60,000 clinical bacterial pathogens isolated in Europe, Turkey, and Israel from 2005 to 2010". Antimicrobial Agents and Chemotherapy 58 (7): 3882–3888. July 2014. doi:10.1128/AAC.02465-14. PMID 24777091.
- ↑ "Activity of ceftobiprole against methicillin-resistant Staphylococcus aureus strains with reduced susceptibility to daptomycin, linezolid or vancomycin, and strains with defined SCCmec types". International Journal of Antimicrobial Agents 43 (4): 323–327. April 2014. doi:10.1016/j.ijantimicag.2013.11.005. PMID 24411474.
- ↑ "A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation". International Journal of Antimicrobial Agents 39 (3): 240–246. March 2012. doi:10.1016/j.ijantimicag.2011.11.005. PMID 22230331.
- ↑ "A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia". Clinical Infectious Diseases 59 (1): 51–61. July 2014. doi:10.1093/cid/ciu219. PMID 24723282.
- ↑ "Zevtera 500 mg powder for concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". 5 April 2023. https://www.medicines.org.uk/emc/product/9164/smpc.
- ↑ "In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci". Antimicrobial Agents and Chemotherapy 45 (3): 825–836. March 2001. doi:10.1128/AAC.45.3.825-836.2001. PMID 11181368.
- ↑ "In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci". The Journal of Antimicrobial Chemotherapy 50 (6): 915–932. December 2002. doi:10.1093/jac/dkf249. PMID 12461013.
 |




