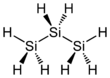
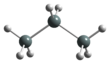
Chemistry:Trisilane
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trisilane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 3194 | ||
| |||
| |||
| Properties | |||
| H8Si3 | |||
| Molar mass | 92.319 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Unpleasant | ||
| Density | 0.743 g cm−3 | ||
| Melting point | −117 °C (−179 °F; 156 K) | ||
| Boiling point | 53 °C (127 °F; 326 K) | ||
| Slowly decomposes[1] | |||
| Vapor pressure | 12.7 kPa | ||
| Hazards | |||
| Main hazards | Pyrophoric | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H250, H261, H315, H319, H335 | |||
| P210, P222, P231+232, P261, P264, P271, P280, P302+334, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P402+404, P403+233, P405, P422, P501 | |||
| Flash point | < −40 °C (−40 °F; 233 K) | ||
| < 50 °C (122 °F; 323 K) | |||
| Related compounds | |||
Related hydrosilicons
|
Disilane Disilyne Silane Silylene | ||
Related compounds
|
Propane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trisilane is the silane with the formula H2Si(SiH3)2. A liquid at standard temperature and pressure, it is a silicon analogue of propane. In contrast with propane, however, trisilane ignites spontaneously in air.[2]
Synthesis
Trisilane was characterized by Alfred Stock having prepared it by the reaction of hydrochloric acid and magnesium silicide.[3][4] This reaction had been explored as early as 1857 by Friedrich Woehler and Heinrich Buff, and further investigated by Henri Moissan and Samuel Smiles in 1902.[2]
Decomposition
The key property of trisilane is its thermal lability. It degrades to silicon films and SiH4 according to this idealized equation:
- Si3H8 → Si + 2 SiH4
In terms of mechanism, this decomposition proceeds by a 1,2 hydrogen shift that produces disilanes, normal and isotetrasilanes, and normal and isopentasilanes.[5]
Because it readily decomposes to leave films of Si, trisilane has been explored a means to apply thin layers of silicon for semiconductors and similar applications.[6] Similarly, thermolysis of trisilane gives silicon nanowires.[7]
References
- ↑ Alfred Walter Stewart (1926) (in English). Recent Advances in Physical and Inorganic Chemistry. Longmans, Green and Company, Limited. pp. 312. https://books.google.com/books?id=o3MVAQAAIAAJ. Retrieved 11 May 2021.
- ↑ 2.0 2.1 P. W. Schenk (1963). "Silanes". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 680.
- ↑ Stock, Alfred; Somieski, Carl (1916). "Siliciumwasserstoffe. I. Die aus Magnesiumsilicid und Säuren entstehenden Siliciumwasserstoffe". Berichte der Deutschen Chemischen Gesellschaft 49: 111–157. doi:10.1002/cber.19160490114. https://zenodo.org/record/1426597.
- ↑ Stock, Alfred; Stiebeler, Paul; Zeidler, Friedrich (1923). "Siliciumwasserstoffe, XVI.: Die höheren Siliciumhydride". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 56 (7): 1695–1705. doi:10.1002/cber.19230560735.
- ↑ Vanderwielen, A. J.; Ring, M. A.; O'Neal, H. E. (1975). "Kinetics of the thermal decomposition of methyldisilane and trisilane". Journal of the American Chemical Society 97 (5): 993–998. doi:10.1021/ja00838a008.
- ↑ United States Patent Application Publication. Pub No. US 2012/0252190 A1, OCT, 4, 2012. Zehavi et al.
- ↑ Heitsch, Andrew T.; Fanfair, Dayne D.; Tuan, Hsing-Yu; Korgel, Brian A. (2008). "Solution−Liquid−Solid (SLS) Growth of Silicon Nanowires". Journal of the American Chemical Society 130 (16): 5436–5437. doi:10.1021/ja8011353. PMID 18373344.
 |