Chemistry:6-MeO-THH
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
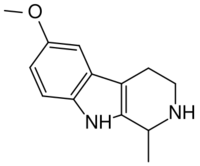
| Formula | C13H16N2O |
| Molar mass | 216.284 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 152–155 °C (306–311 °F) |
| |
| |
| | |
6-MeO-THH, or 6-methoxy-1,2,3,4-tetrahydroharman, is a β-carboline (or more specifically a pinoline) derivative and a structural isomer of tetrahydroharmine (7-MeO-THH). 6-MeO-THH is mentioned in Alexander Shulgin's book TiHKAL (Tryptamines I Have Known and Loved), stating that 6-MeO-THH is very similar to the other carbolines.[1] Limited testing suggests that it possesses mild psychoactive effects at 1.5 mg/kg and is said to be about one-third as potent as 6-methoxyharmalan.[2] It has been isolated from certain plants of the Virola family.
Pharmacology
Very little is known about the psychoactivity of 6-MeO-THH in humans. Studies in rats have shown it to bind to a number of serotonin 5-HT1 receptors and 5-HT2 receptors, dopamine D2 receptors, benzodiazepine receptors, and imidazoline receptors.[2][3][4]
See also
- Beta-carboline
- Harmala alkaloid
- Tryptamine
References
- ↑ Shulgin, Alexander; Shulgin, Ann (September 1997). TiHKAL: The Continuation. Berkeley, California: Transform Press, 425. ISBN 0-9630096-9-9. OCLC 38503252. http://www.erowid.org/library/books_online/tihkal/tihkal.shtml.
- ↑ 2.0 2.1 "Investigation of hallucinogenic and related beta-carbolines". Drug and Alcohol Dependence 50 (2): 99–107. April 1998. doi:10.1016/S0376-8716(97)00163-4. PMID 9649961.
- ↑ "Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors". Drug and Alcohol Dependence 60 (2): 121–32. August 2000. doi:10.1016/S0376-8716(99)00148-9. PMID 10940539.
- ↑ "beta-carboline binding to imidazoline receptors". Drug and Alcohol Dependence 64 (2): 203–8. October 2001. doi:10.1016/S0376-8716(01)00123-5. PMID 11543990.
 |

