Chemistry:Aeruginascin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
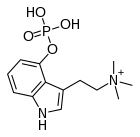
| Formula | C13H20N2O4P |
| Molar mass | 299.287 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Aeruginascin or N,N,N-trimethyl-4-phosphoryloxytryptamine is an indoleamine derivative which occurs naturally within the mushrooms Inocybe aeruginascens[1][2][3][4][5][6] and Pholiotina cyanopus,[6] and Psilocybe cubensis.[7] Aeruginascin is the N-trimethyl analogue of psilocybin. It is closely related to the frog skin toxin bufotenidine (5-HTQ), a potent 5-HT3 receptor agonist, but the aeruginascin metabolite 4-HO-TMT shows strong binding at the 5-HT2 receptors similar to psilocin.[8][9] The first scientific literature about the pharmacological effects of aeruginascin is from a study published by Gartz in 1989.[10] Across 23 analyzed cases of accidental hallucinogenic mushroom poisonings, people who had ingested the mushroom Inocybe aeruginascens reported only euphoric experiences.[11] This is in contrast to the slight and in some cases extremely dysphoric experiences reported from the accidental ingestion of non aeruginascin containing mushrooms (containing solely psilocybin and psilocin).
References
- ↑ "Inocybe aeruginascens Babos.". Eleusis, Journal of Psychoactive Plants & Compounds. (Museo Civico di Rovereto) 3: 31–4. 1995. http://www.museocivico.rovereto.tn.it/context.jsp?ID_LINK=111258&area=279.
- ↑ "Aeruginascin, a trimethylammonium analogue of psilocybin from the hallucinogenic mushroom Inocybe aeruginascens". Planta Medica 72 (7): 665–666. June 2006. doi:10.1055/s-2006-931576. PMID 16673333. http://wwwuser.gwdg.de/~ucoc/laatsch/168_Aeruginascin_col.pdf.
- ↑ "Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin". Journal of Natural Products 83 (2): 461–467. February 2020. doi:10.1021/acs.jnatprod.9b01061. PMID 32077284.
- ↑ "N-methylated tryptamine derivatives in citrus genus plants: identification of N,N,N-trimethyltryptamine in bergamot". Journal of Agricultural and Food Chemistry 60 (37): 9512–9518. September 2012. doi:10.1021/jf302767e. PMID 22957740.
- ↑ "Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra". Molecules 24 (6): 1026. March 2019. doi:10.3390/molecules24061026. PMID 30875889.
- ↑ 6.0 6.1 "Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids". International Journal of Molecular Sciences 23 (22): 14068. November 2022. doi:10.3390/ijms232214068. PMID 36430546.
- ↑ "CaaMTech Publishes Fundamental Research on Aeruginascin Derivatives". 14 September 2022. https://caam.tech/caamtech-publishes-fundamental-research-on-aeruginascin-derivatives/.
- ↑ "Active Metabolite of Aeruginascin (4-Hydroxy-N,N,N-trimethyltryptamine): Synthesis, Structure, and Serotonergic Binding Affinity". ACS Omega 5 (27): 16940–16943. July 2020. doi:10.1021/acsomega.0c02208. PMID 32685863.
- ↑ "Study Finds Aeruginascin Metabolite 4-HO-TMT is Active at the Serotonin 5-HT2A Receptor" (in en-US). 2020-07-07. https://psychedelicreview.com/study-finds-aeruginascin-metabolite-4-ho-tmt-is-active-at-the-serotonin-5-ht2a-receptor/.
- ↑ "Analysis of Aeruginascin in Fruit Bodies of the Mushroom Inocybe aeruginascens" (in en). International Journal of Crude Drug Research 27 (3): 141–144. January 1989. doi:10.3109/13880208909053954. ISSN 0167-7314. http://www.tandfonline.com/doi/full/10.3109/13880208909053954.
- ↑ "Aeruginascin" (in en-US). 2018-11-19. https://psychedelicreview.com/compound/aeruginascin/.
 |


