Chemistry:FGIN-143
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
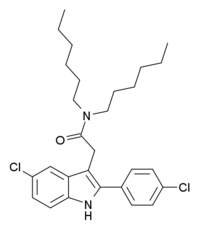
| Formula | C28H36Cl2N2O |
| Molar mass | 487.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
FGIN-1-43 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone, and is several times more potent than the related drug FGIN-127.[1][2][3][4]
References
- ↑ "2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR)". The Journal of Pharmacology and Experimental Therapeutics 262 (3): 971–8. September 1992. PMID 1326631.
- ↑ "Chemistry, binding affinities, and behavioral properties of a new class of "antineophobic" mitochondrial DBI receptor complex (mDRC) ligands". Journal of Medicinal Chemistry 36 (20): 2908–20. October 1993. doi:10.1021/jm00072a010. PMID 8411007.
- ↑ "The pharmacology of neurosteroidogenesis". The Journal of Steroid Biochemistry and Molecular Biology 49 (4–6): 385–9. June 1994. doi:10.1016/0960-0760(94)90284-4. PMID 8043504.
- ↑ "Synthesis and preliminary behavioural evaluation in mice of new 3-aryl-3-pyrrol-1-ylpropanamides, analogues of FGIN-1-27 and FGIN-1-43". The Journal of Pharmacy and Pharmacology 53 (11): 1561–8. November 2001. doi:10.1211/0022357011777945. PMID 11732760.
 |
