Chemistry:Methylprednisolone acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Depo-Medrol, Depo-Medrate, Depo-Medrone, others |
| Other names | Depot methylprednisolone acetate; Methylprednisolone 21-acetate; 6α-Methylprednisolone 21-acetate; NSC-48985 |
| Routes of administration | Intramuscular injection |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
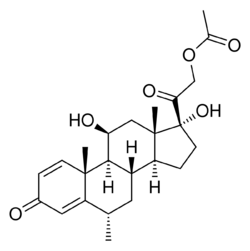
| Formula | C24H32O6 |
| Molar mass | 416.514 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Methylprednisolone acetate, sold under the brand names Depo-Medrol among others, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester—specifically the C21 acetate ester of methylprednisolone—which is used in clinical and veterinary medicine.[1][2][3][4][5][6][7] It has been formulated as an aqueous suspension for intramuscular, intra-articular, soft tissue, and intralesional injection alone and in combination with lidocaine, a local anesthetic.[1][4][5] Methylprednisolone acetate was previously suspended with polyethylene glycol but is no longer formulated with this excipient due to concerns about possible toxicity.[6][8] Depo methylprednisolone acetate is a depot injection and is absorbed slowly with a duration of weeks to months with a single intramuscular injection.[5]
See also
References
- ↑ 1.0 1.1 "Depo-Medrol- methylprednisolone acetate injection, suspension". 18 January 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9a7b3837-e038-48bf-97e9-78ad463760dc.
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 811–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA811.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 675–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA675.
- ↑ 4.0 4.1 European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. 19 June 1998. pp. 337–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA337.
- ↑ 5.0 5.1 5.2 Plumb's Veterinary Drug Handbook: Pocket. John Wiley & Sons. 21 February 2018. pp. 1088–1091. ISBN 978-1-119-34649-4. https://books.google.com/books?id=wshQDwAAQBAJ&pg=PA1088.
- ↑ 6.0 6.1 Pain Procedures in Clinical Practice E-Book. Elsevier Health Sciences. 11 June 2011. pp. 8–. ISBN 978-1-4377-3774-5. https://books.google.com/books?id=JNpNeW05rLIC&pg=PA8.
- ↑ Equine Dermatology - E-Book. Elsevier Health Sciences. 1 December 2010. pp. 122–. ISBN 978-1-4377-0921-6. https://books.google.com/books?id=f0-v1vOq3x4C&pg=PA122.
- ↑ Campbell's Operative Orthopaedics E-Book. Elsevier Health Sciences. 29 October 2012. pp. 1906–. ISBN 978-0-323-08718-6. https://books.google.com/books?id=1Bvfn-1ZRWgC&pg=PA1906.
 |

