Biology:Oprozomib
 | |
| Clinical data | |
|---|---|
| Pronunciation | /oʊˈprɒzoʊmɪb/ oh-PROZ-oh-mib |
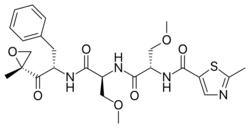
| Other names | O-methyl-N-(2-methyl-1,3-thiazol-5-carbonyl)-L-seryl-O-methyl-N-{(2S)-1-[(2R)-2-methyloxiran-2-yl]-1-oxo-3-phenylpropan-2-yl}-L-serinamide |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H32N4O7S |
| Molar mass | 532.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Oprozomib[1] (codenamed ONX 0912 and PR-047) is an orally active second-generation proteasome inhibitor developed by Proteolix, which was acquired by Onyx Pharmaceuticals, an Amgen subsidiary, in 2009. It selectively inhibits chymotrypsin-like activity of both the constitutive proteasome (PSMB5) and immunoproteasome (LMP7).[2]
It is being investigated for the treatment of hematologic malignancies, specifically, multiple myeloma, with Phase 1b studies ongoing (as of February 16, 2016).[3] Being an epoxyketone derivative, oprozomib is structurally related to carfilzomib and has the added benefit of being orally bioavailable. Like carfilzomib, it is active against bortezomib-resistant multiple myeloma cells.[4]
Oprozomib was granted orphan drug status for the treatment of Waldenström's macroglobulinaemia and multiple myeloma in 2014.[5]
See also
- Ixazomib (trade name Ninlaro) — an orally available boronic acid-derived proteasome inhibitor approved for the treatment of multiple myeloma
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed International Nonproprietary Names: List 107". World Health Organization. p. 193. https://www.who.int/medicines/publications/druginformation/innlists/PL107.pdf.
- ↑ "Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047)". Journal of Medicinal Chemistry 52 (9): 3028–38. May 2009. doi:10.1021/jm801329v. PMID 19348473.
- ↑ "Amgen Pipeline Chart". Amgen Inc.. February 16, 2016. p. 3. http://www.amgenpipeline.com/~/media/amgen/full/www-amgenpipeline-com/charts/amgenpipelinedownload.ashx.
- ↑ "A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma". Blood 116 (23): 4906–15. December 2010. doi:10.1182/blood-2010-04-276626. PMID 20805366.
- ↑ "Oprozomib - Onyx Pharmaceuticals". Adis Insight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800032242.
 |

