Chemistry:Filgotinib
 | |
| Clinical data | |
|---|---|
| Trade names | Jyseleca |
| Other names | GLPG0634, GS-6034[1] |
| License data | |
| Routes of administration | By mouth |
| Drug class | Janus kinase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 6 hours[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
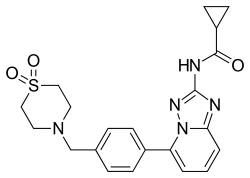
| Formula | C21H23N5O3S |
| Molar mass | 425.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA).[3] It was developed by the Belgian-Dutch biotech company Galapagos NV.[6]
The most common side effects include nausea (feeling sick), upper respiratory tract infection (nose and throat infection), urinary tract infection and dizziness.[3]
Filgotinib was approved for medical use in both the European Union and Japan in September 2020.[3][6][7]
Medical uses
Filgotinib is indicated for the treatment of moderate to severe active rheumatoid arthritis in adults who have responded inadequately to, or who are intolerant to one or more disease‑modifying anti‑rheumatic drugs (DMARDs).[3] Filgotinib may be used as monotherapy or in combination with methotrexate (MTX).[3]
Mechanism of action
Filgotinib is a Janus kinase inhibitor with selectivity for subtype JAK1 of this enzyme. It is considered a promising agent as it inhibits JAK1 selectively, similar to already marketed upadacitinib. Less selective JAK inhibitors (e.g. tofacitinib and baricitinib) are already being marketed. They show long-term efficacy in the treatment of various inflammatory diseases. However, their lack of selectivity leads to dose-limiting side effects.[5] It is thought that inhibition of all JAK isoenzymes is beneficial in rheumatoid arthritis. However, pan-JAK inhibition might also lead to unwanted side effects that might not outweigh its benefits. This is the rationale for the development of newer and more selective inhibitors like filgotinib.
The signal transmission of large numbers of proinflammatory cytokines is dependent on JAK1. Inhibition of JAK2 may also contribute to the efficacy against rheumatoid arthritis. Nonetheless it is thought that JAK2 inhibition might lead to anemia and thrombopenia by interference with erythropoietin and thrombopoietin and granulocyte-macrophage colony-stimulating factor. Therefore, one might prefer to choose a more selective JAK1 inhibitor as a primary therapeutic option. Filgotinib exerts a 30-fold selectivity for JAK1 compared to JAK2.[8] It is however still to be seen to what extent JAK2 inhibition should be avoided.
History
- June 2011: results of first Phase II trial[citation needed]
- November 2014: initiation of DARWIN 1 and 2 trials[citation needed]
- July 2015: DARWIN 1 results released[citation needed]
- August 2015: DARWIN 2 trial results released[citation needed]
- September 2015: AbbVie opted out of collaboration with Galapagos[9]
- December 2015: Galapagos signed partnership with Gilead to co-develop & co-commercialize Filgotinib for various diseases
- December 2019: Gilead submitted a New Drug Application (NDA) with a priority review voucher to the U.S. Food and Drug Administration (FDA) for filgotinib.[10]
- On 23 July 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Jyseleca, intended for the treatment of rheumatoid arthritis.[11] The applicant for this medicinal product is Gilead Sciences Ireland UC.[11][12][13]
- 19 August 2020: FDA rejects Gilead’s filing for approval of filgotinib over toxicity concerns[14]
- September 2020: Filgotinib was approved for medical use in both the European Union and Japan.[3][6][7]
Research
Clinical trials
The efficacy of filgotinib is being studied in a Phase IIb program (DARWIN trial 1, 2) with involvement of 886 rheumatoid arthritis patients and 180 Crohn's disease patients.[citation needed]
Phase I study
It was shown in Phase I studies that the pharmacokinetics of filgotinib metabolism is independent of hepatic CYP450 enzymatic degradation. The drug metabolism is however mediated by carboxylesterases. There is no interference reported with the metabolism of methotrexate nor with any of the investigated transport proteins.[15]
Phase II study: Proof of concept (2011)
In November 2011 Galapagos released the results of their Phase II study (identification: NCT01384422, Eudract: 2010-022953-40) in which 36 rheumatoid arthritis patients were treated who showed a suboptimal clinical response to methotrexate treatment.[16][full citation needed] Three groups of twelve patients were treated either with 200 mg filgotinib in a single dose, 200 mg divided in two doses or placebo. The primary end-point was the ACR20 score, which monitors improvements in the symptomatology of the patient. After the scheduled 4 weeks of treatment, 83% of the respondents showed an improved ACR20-score. Half of the treated patients showed a complete (or near complete) remission of the disease. There were no reports of anemia nor changes in lipidemia. The company stated in their press release that filgotinib is the first selective JAK1 inhibitor that shows clinical efficacy. As a result of this study, the company stated that "GLPG0634 shows one of the highest initial response rates ever reported for rheumatoid arthritis treatments".[17]
DARWIN 1 trial
The DARWIN 1 trial was a 24-week double blind placebo-controlled trial with 599 rheumatoid arthritis patients enrolled. All participants had moderate to severe rheumatoid arthritis and showed an insufficient response to standard methotrexate treatment. The trial compared three dosages of filgotinib as a once or twice per day regimen.[18][full citation needed] During the trial all participants remained on their methotrexate treatment. The trial completed in Feb 2015 and the results were released in July 2015.[19][20] Galapagos announced that the drug met key efficacy endpoints, showed ACR70 responses up to 39%, and maintained its safety profile.[20][21]
DARWIN 2 trial
The DARWIN 2 trial was a double blind placebo-controlled trial with 280 rheumatoid arthritis patients enrolled who show an insufficient response to standard methotrexate treatment. In contrast to the previous DARWIN 1 trial, methotrexate was discontinued. Therefore, this trial investigates filgotinib as a second-line monotherapy.[22] The recruitment of DARWIN trial 2b ended in November 2014.[23] In August 2015, Galapagos announced that the study confirmed previous results.[24]
DARWIN 3 trial
Patients who completed DARWIN 1 and 2 were eligible for DARWIN 3. In November 2017, the company announced consistent safety findings and durable activity at week 84 in the trial.[25] The estimated study completion timeframe is May 2019.[26][full citation needed]
FINCH Phase III trials
FINCH 1 looks at patients where first-line treatment with methotrexate (MTX) is not working. It compares filgotinib versus adalimumab/Humira versus a placebo.[27] FINCH 2 looks at patients where a biologic is not working. FINCH 3 looks at filgotinib as a first-line treatment unlike previous studies that investigated the drug as a second-line treatment.
FINCH 2 trial revealed patients with active rheumatoid arthritis who had an inadequate response or intolerance to one or more DMARDs, filgotinib showed significance in treatment response compared with placebo.[28]
MANTA
Due to concerns over testicular toxicity in males, the MANTA study is examining the safety of the drug in the context of treating ulcerative colitis.[29][full citation needed] Despite these concerns, the FDA allowed a 200-mg daily dose for males in the Phase III FINCH trials.[30]
References
- ↑ "Pipeline". Gilead Sciences. 27 July 2020. https://www.gilead.com/science-and-medicine/pipeline.
- ↑ "Jyseleca 100 mg film-coated tablets - Summary of Product Characteristics (SmPC)". 1 October 2020. https://www.medicines.org.uk/emc/product/11809/smpc.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Jyseleca EPAR". 26 May 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/jyseleca. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Jyseleca Product information". https://ec.europa.eu/health/documents/community-register/html/h1480.htm.
- ↑ 5.0 5.1 "Pharmacokinetics and Pharmacokinetic/Pharmacodynamic Modeling of Filgotinib (GLPG0634), a Selective JAK1 Inhibitor, in Support of Phase IIB Dose Selection". Clinical Pharmacokinetics 54 (8): 859–74. August 2015. doi:10.1007/s40262-015-0240-z. PMID 25681059.
- ↑ 6.0 6.1 6.2 "European Commission Grants Marketing Authorization for Jyseleca (Filgotinib) for the Treatment of Adults With Moderate to Severe Active Rheumatoid Arthritis" (Press release). Gilead Sciences. 25 September 2020. Retrieved 4 October 2020 – via Business Wire.
- ↑ 7.0 7.1 "Jyseleca (Filgotinib) Approved in Japan for Rheumatoid Arthritis". Gilead Sciences. 25 September 2020. https://www.businesswire.com/news/home/20200924005942/en/.
- ↑ "Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases". Journal of Immunology 191 (7): 3568–77. October 2013. doi:10.4049/jimmunol.1201348. PMID 24006460.
- ↑ "AbbVie to Advance Once-Daily ABT-494 to Phase 3 in Rheumatoid Arthritis by Year-End". AbbVie (Press release).
- ↑ "Gilead Submits Filgotinib New Drug Application to U.S. Food and Drug Administration Under Priority Review for Rheumatoid Arthritis Treatment". Gilead Sciences, Inc. (Press release). 19 December 2019. Retrieved 27 July 2020.
- ↑ 11.0 11.1 "Jyseleca: Pending EC decision". 23 July 2020. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/jyseleca. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Gilead and Galapagos Announce Positive European CHMP Opinion for Jyseleca (Filgotinib) for the Treatment of Adults With Moderate to Severe Rheumatoid Arthritis" (Press release). 24 July 2020. Retrieved 27 July 2020.
- ↑ "Gilead and Galapagos Announce Positive European CHMP Opinion for Jyseleca (Filgotinib) for the Treatment of Adults With Moderate to Severe Rheumatoid Arthritis". Gilead Sciences, Inc. (Press release). 24 June 2020. Retrieved 27 July 2020.
- ↑ "FDA rejects Gilead's would-be blockbuster filgotinib over toxicity concerns". 19 August 2020. https://www.fiercebiotech.com/biotech/fda-rejects-gilead-s-would-be-blockbuster-filgotinib-over-toxicity-concerns.
- ↑ "Phase 1 and Phase 2 Data Confirm That GLPG0634, a Selective JAK1 Inhibitor, Has a Low Potential for Drug-Drug Interactions". 2014 ACR/ARHP Annual Meeting. American College of Rheumatology. 2014. 1481. http://acrabstracts.org/abstracts/phase-1-and-phase-2-data-confirm-that-glpg0634-a-selective-jak1-inhibitor-has-a-low-potential-for-drug-drug-interactions/.
- ↑ Clinical trial number NCT01384422 for "Safety and Preliminary Efficacy of GLPG0634 in Methotrexate-refractory Active Rheumatoid Arthritis" at ClinicalTrials.gov
- ↑ "Galapagos' GLPG0634 shows excellent efficacy and safety in rheumatoid arthritis Phase II study" (PDF) (Press release). Retrieved 26 February 2015.
- ↑ Clinical trial number NCT01888874 for "Dose-finding Study of GLPG0634 as add-on to Methotrexate in Active Rheumatoid Arthritis Patients (DARWIN1)" at ClinicalTrials.gov
- ↑ "Galapagos reports that the last patient in DARWIN 1 has completed 12 weeks of treatment" (PDF) (Press release). Retrieved 26 February 2015.
- ↑ 20.0 20.1 "Galapagos' selective JAK1 inhibitor filgotinib meets key efficacy endpoints, shows ACR70 responses up to 39%, and maintains safety profile after 24 weeks of treatment in DARWIN 1 Phase 2B study" (Press release). Galapagos NV. 29 July 2015 – via GlobeNewswire.
- ↑ "Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1)". Annals of the Rheumatic Diseases 76 (6): 998–1008. June 2017. doi:10.1136/annrheumdis-2016-210104. PMID 27993829.
- ↑ "Galapagos completes recruitment for Darwin 1 study with GLPG0634 (filgotinib) in RA" (Press release). Galapagos NV. Archived from the original on 26 February 2015. Retrieved 26 February 2015 – via GlobeNewswire.
- ↑ "Galapagos completes recruitment for Darwin 2 monotherapy study with GLPG0634 (filgotinib) in RA" (Press release). Galapagos NV. 24 November 2014. Retrieved 26 February 2015 – via GlobeNewswire.
- ↑ "DARWIN 2 24-week monotherapy data in RA confirm previous results and support best-in-class potential for filgotinib" (Press release). Galapagos NV. 10 August 2015 – via GlobeNewswire.
- ↑ "Consistent safety findings and durable activity with filgotinib treatment of rheumatoid arthritis patients up to week 84 in DARWIN 3 study" (Press release). Galapagos NV. 5 November 2017 – via GlobeNewswire.
- ↑ Clinical trial number NCT02065700 for "Long-term Follow-up Study of GLPG0634 in Active Rheumatoid Arthritis Patients" at ClinicalTrials.gov
- ↑ "Filgotinib program in RA - Galapagos Annual Report 2016". http://reports.glpg.com/annual-report-2016/en/r-d/rheumatoid-arthritis/filgotinib-program-in-ra.html.
- ↑ "Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial". JAMA 322 (4): 315–325. July 2019. doi:10.1001/jama.2019.9055. PMID 31334793.
- ↑ Clinical trial number NCT03201445 for "Study to Evaluate the Testicular Safety of Filgotinib in Adult Males With Moderately to Severely Active Ulcerative Colitis" at ClinicalTrials.gov
- ↑ "Galapagos, Gilead include high dose in PhIII RA trial after talk with FDA". https://www.fiercebiotech.com/biotech/galapagos-gilead-include-high-dose-phiii-ra-trial-following-talk-fda.
External links
- Clinical trial number NCT02889796 for "Filgotinib in Combination With Methotrexate in Adults With Moderately to Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Methotrexate (FINCH 1)" at ClinicalTrials.gov
- Clinical trial number NCT02873936 for "Filgotinib Versus Placebo in Adults With Active Rheumatoid Arthritis (RA) Who Have an Inadequate Response to Biologic Disease-modifying Anti-rheumatic Drug(s) (DMARDs) Treatment (FINCH 2)" at ClinicalTrials.gov
- Clinical trial number NCT02886728 for "Filgotinib Alone and in Combination With Methotrexate (MTX) in Adults With Moderately to Severely Active Rheumatoid Arthritis Who Are Naive to MTX Therapy (FINCH 3)" at ClinicalTrials.gov
- Clinical trial number NCT02914522 for "Filgotinib in the Induction and Maintenance of Remission in Adults With Moderately to Severely Active Ulcerative Colitis (SELECTION1)" at ClinicalTrials.gov
- Clinical trial number NCT02914561 for "Filgotinib in the Induction and Maintenance of Remission in Adults With Moderately to Severely Active Crohn's Disease (Diversity1)" at ClinicalTrials.gov
- Clinical trial number NCT04115748 for "Study to Evaluate the Efficacy and Safety of Filgotinib in Participants With Active Psoriatic Arthritis Who Are Naive to Biologic DMARD Therapy (PENGUIN 1)" at ClinicalTrials.gov
- Clinical trial number NCT04115839 for "Study to Evaluate the Efficacy and Safety of Filgotinib in Participants With Active Psoriatic Arthritis Who Have an Inadequate Response or Are Intolerant to Biologic DMARD Therapy (PENGUIN 2)" at ClinicalTrials.gov
 |

