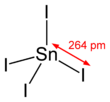Chemistry:Tin(IV) iodide
|
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
tin(IV) iodide
| |||
| Other names
tin tetraiodide
stannic iodide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| SnI4 | |||
| Molar mass | 626.328 g mol−1 | ||
| Appearance | red-orange solid | ||
| Density | 4.56 g cm−3 | ||
| Melting point | 143 °C (289 °F; 416 K) | ||
| Boiling point | 348.5 °C (659.3 °F; 621.6 K) | ||
Refractive index (nD)
|
2.106 | ||
| Structure | |||
| Cubic, cP40 | |||
| Pa-3 No. 205 | |||
| Related compounds | |||
Other anions
|
Tin(IV) fluoride Tin(IV) chloride Tin(IV) bromide | ||
Other cations
|
Carbon tetraiodide Silicon tetraiodide Germanium tetraiodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
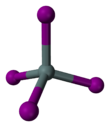
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as benzene.[1]
Preparation
The compound is usually prepared by the reaction of iodine and tin:[2]
- Sn + 2I
2 → SnI
4
Chemical properties
The compound hydrolyses in water.[3] In aqueous hydroiodic acid, it reacts to form a rare example of a hexaiodometallate:[2]
- SnI4 + 2 I− → [SnI6]2−
Physical properties
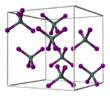
Tin(IV) iodide is an orange solid under standard conditions.[3] It has a cubic crystal structure with the space group Pa3 (space group no. 205), the lattice parameter a = 1226 pm and eight formula units per unit cell.[4] This corresponds approximately to a cubic close packing of iodine atoms in which 1/8 of all tetrahedral gaps are occupied by tin atoms. This leads to discrete tetrahedral SnI4 molecules.[5]
See also
References
- ↑ Chemistry : Periodic Table : tin : compound data [tin (IV) iodide]
- ↑ 2.0 2.1 Moeller, T.; Edwards, D. C. (1953). "Tin(IV) Iodide (Stannic Iodide)". Inorganic Syntheses 4: 119–121. doi:10.1002/9780470132357.ch40.
- ↑ 3.0 3.1 Hickling, George G. (Aug 1990). "Gravimetric analysis: The synthesis of tin iodide" (in en). Journal of Chemical Education 67 (8): 702. doi:10.1021/ed067p702. ISSN 0021-9584. https://pubs.acs.org/doi/abs/10.1021/ed067p702.
- ↑ Meller, F.; Fankuchen, I. (1955-06-10). "The crystal structure of tin tetraiodide" (in en). Acta Crystallographica 8 (6): 343–344. doi:10.1107/S0365110X55001035. ISSN 0365-110X. https://scripts.iucr.org/cgi-bin/paper?S0365110X55001035.
- ↑ Wiberg, Egon; Wiberg, Nils (2007). Holleman, Arnold F.. ed. Lehrbuch der anorganischen Chemie (102., stark umgearbeitete und verbesserte Auflage ed.). Berlin New York: Walter de Gruyter. ISBN 978-3-11-017770-1.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |