Chemistry:Metformin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /mɛtˈfɔːrmɪn/, met-FOR-min |
| Trade names | Fortamet, Glucophage, Glumetza, others |
| Other names | N,N-dimethylbiguanide[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696005 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50–60%[8][9] |
| Protein binding | Minimal[8] |
| Metabolism | Not by liver[8] |
| Elimination half-life | 4–8.7 hours[8] |
| Excretion | Urine (90%)[8] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C4H11N5 |
| Molar mass | 129.167 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.3±0.1[10] g/cm3 |
| |
| |
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of type 2 diabetes,[11][12][13][14] particularly in people who are overweight.[12] It is also used in the treatment of polycystic ovary syndrome.[13] It is sometimes used as an off-label adjunct to lessen the risk of metabolic syndrome in people who take antipsychotics.[15] Metformin is not associated with weight gain[16] and is taken by mouth.[13]
Metformin is generally well tolerated.[17] Common adverse effects include diarrhea, nausea, and abdominal pain.[13] It has a small risk of causing low blood sugar.[13] High blood lactic acid level (acidosis) is a concern if the medication is used in overly large doses or prescribed in people with severe kidney problems.[18][19]
Metformin is a biguanide anti-hyperglycemic agent.[13] It works by decreasing glucose production in the liver, increasing the insulin sensitivity of body tissues,[13] and increasing GDF15 secretion, which reduces appetite and caloric intake.[20][21][22][23]
Metformin was first described in scientific literature in 1922 by Emil Werner and James Bell.[24] French physician Jean Sterne began the study in humans in the 1950s.[24] It was introduced as a medication in France in 1957 and the United States in 1995.[13][25] Metformin is on the World Health Organization's List of Essential Medicines,[26] and is the most widely used medication for diabetes taken by mouth.[24] It is available as a generic medication.[13] In 2021, it was the second most commonly prescribed medication in the United States, with more than 91 million prescriptions.[27][28]
Medical uses
Metformin is used to lower the blood glucose in those with type 2 diabetes.[13] It is also used as a second-line agent for infertility in those with polycystic ovary syndrome.[13][29]
Type 2 diabetes
The American Diabetes Association and the American College of Physicians both recommend metformin as a first-line agent to treat type 2 diabetes.[30][31][32] It is as effective as repaglinide and more effective than all other oral drugs for type 2 diabetes.[33]
Efficacy
Treatment guidelines for major professional associations, including the European Association for the Study of Diabetes, the European Society for Cardiology, and the American Diabetes Association, describe evidence for the cardiovascular benefits of metformin as equivocal.[31][34] A 2020 Cochrane systematic review did not find enough evidence of reduction of cardiovascular mortality, non-fatal myocardial infarction or non-fatal stroke when comparing metformin monotherapy to other glucose-lowering drugs, behaviour change interventions, placebo or no intervention.[35]
The use of metformin reduces body weight in people with type 2 diabetes[20][36] in contrast to sulfonylureas, which are associated with weight gain.[36] Some evidence shows that metformin is associated with weight loss in obesity in the absence of diabetes.[37][38] Metformin has a lower risk of hypoglycemia than the sulfonylureas,[39][40] although hypoglycemia has uncommonly occurred during intense exercise, calorie deficit, or when used with other agents to lower blood glucose.[41][42] Metformin modestly reduces low density lipoprotein and triglyceride levels.[39][40]
In individuals with prediabetes, a 2019 systematic review comparing the effects of metformin with other interventions in the reduction of risk of developing type 2 diabetes[43] found moderate-quality evidence that metformin reduced the risk of developing type 2 diabetes when compared to diet and exercise or a placebo.[43] However, when comparing metformin to intensive diet or exercise, moderate-quality evidence was found that metformin did not reduce risk of developing type 2 diabetes and very low-quality evidence was found that adding metformin to intensive diet or exercise did not show any advantage or disadvantage in reducing risk of type 2 diabetes when compared to intensive exercise and diet alone.[43] The same review also found one suitable trial comparing the effects of metformin and sulfonylurea in reducing risk of developing type 2 diabetes in prediabetic individuals, however this trial did not report any patient relevant outcomes.[43]
Polycystic ovarian syndrome
In those with polycystic ovarian syndrome (PCOS), tentative evidence shows that metformin use increases the rate of live births.[44] This includes in those who have not been able to get pregnant with clomiphene.[45] Metformin does not appear to change the risk of miscarriage.[44] A number of other benefits have also been found both during pregnancy and in nonpregnant women with PCOS.[46][47] In an updated Cochrane (2020) review on metformin versus placebo/no treatment before or during IVF/ICSI in women with PCOS no conclusive evidence of improved live birth rates was found.[48] In long GnRH-agonist protocols there was uncertainty in the evidence of improved live birth rates but there could be increases in clinical pregnancy rate.[48] In short GnRH-antagonist protocols metformin may reduce live birth rates with uncertainty on its effect on clinical pregnancy rate.[48] Metformin may result in a reduction of OHSS but could come with a greater frequency of side effects.[48] There was uncertainty as to metformin's impact on miscarriage.[48] The evidence does not support general use during pregnancy for improving maternal and infant outcomes in obese women.[49]
The United Kingdom's National Institute for Health and Clinical Excellence recommended in 2004 that women with PCOS and a body mass index above 25 be given metformin for anovulation and infertility when other therapies fail to produce results.[50] UK and international clinical practice guidelines do not recommend metformin as a first-line treatment[51] or do not recommend it at all, except for women with glucose intolerance.[52] The guidelines suggest clomiphene as the first medication option and emphasize lifestyle modification independently from medical treatment. Metformin treatment decreases the risk of developing type 2 diabetes in women with PCOS who exhibited impaired glucose tolerance at baseline.[53][54]
Gastric Cancer
Gastric cancer (GC) stands as a major global health concern due to its high prevalence and mortality rate. Amidst various treatment avenues, metformin, a common medication for type-2 diabetes mellitus (T2DM), has garnered attention for its potential anti-cancer properties. While its effectiveness in combating GC has been a subject of debate, recent clinical studies predominantly support metformin's protective impact on reducing the risk and improving the survival rates of GC patients.[55] The drug's anti-cancer effects are believed to be mediated through multiple pathways, particularly involving AMPK activation and IGF-1R modulation. Despite promising findings, the consensus on metformin's application in GC prevention and treatment necessitates further clinical and mechanistic studies to confirm its therapeutic role.[56]
Diabetes and pregnancy
A total review of metformin use during pregnancy compared to insulin alone found good short-term safety for both the mother and baby, but unclear long-term safety.[57] Several observational studies and randomized controlled trials found metformin to be as effective and safe as insulin for the management of gestational diabetes.[58][59] Nonetheless, several concerns have been raised and evidence on the long-term safety of metformin for both mother and child is lacking.[60] Compared with insulin, women with gestational diabetes treated with metformin gain less weight and are less likely to develop pre‐eclampsia during pregnancy.[60][61] Babies born to women treated with metformin have less visceral fat, and this may make them less prone to insulin resistance in later life.[62] The use of metformin for gestational diabetes resulted in smaller babies compared to treatment with insulin. However, despite initially lower birth weight, children exposed to metformin during pregnancy had accelerated growth after birth, and were heavier by mid-childhood than those exposed to insulin during pregnancy. This pattern of initial low birth weight followed by catch-up growth that surpasses comparative children has been associated with long-term cardiometabolic disease.[63]
Weight change
Metformin use is typically associated with weight loss.[64] It appears to be safe and effective in counteracting the weight gain caused by the antipsychotic medications olanzapine and clozapine.[65][66] Although modest reversal of clozapine-associated weight gain is found with metformin, primary prevention of weight gain is more valuable.[67]
Use with insulin
Metformin may reduce the insulin requirement in type 1 diabetes, albeit with an increased risk of hypoglycemia.[68]
Life extension
There is some evidence metformin may be helpful in extending lifespan, even in otherwise healthy people. It has received substantial interest as an agent that delays aging, possibly through similar mechanisms as its treatment of diabetes (insulin and carbohydrate regulation).[69][70]
Alzheimer's disease
The possibility that metformin can delay the onset or progression of Alzheimer's disease, or even prevent it, is under investigation. There has been a variety of research published in this field in an effort to establish a correlation between type 2 diabetes and Alzheimer's disease.[71] Research includes studies with participants not diagnosed with diabetes.[72]
Contraindications
Metformin is contraindicated in people with:
- Severe renal impairment (estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2)[73]
- Known hypersensitivity to metformin[73]
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis (from uncontrolled diabetes),[73] with or without coma[74]
Adverse effects
The most common adverse effect of metformin is gastrointestinal irritation, including diarrhea, cramps, nausea, vomiting, and increased flatulence. Metformin is more commonly associated with gastrointestinal adverse effects than most other antidiabetic medications.[40] The most serious potential adverse effect of metformin is lactic acidosis; this complication is rare, and seems to be related to impaired liver or kidney function.[75] Metformin is not approved for use in those with severe kidney disease, but may still be used at lower doses in those with kidney problems.[76]
Gastrointestinal
Gastrointestinal upset can cause severe discomfort; it is most common when metformin is first administered, or when the dose is increased.[74] The discomfort can often be avoided by beginning at a low dose (1.0 to 1.7 g/day) and increasing the dose gradually, but even with low doses, 5% of people may be unable to tolerate metformin.[74][77] Use of slow or extended-release preparations may improve tolerability.[77]
Long-term use of metformin has been associated with increased homocysteine levels[78] and malabsorption of vitamin B12.[74][79][80] Higher doses and prolonged use are associated with increased incidence of vitamin B12 deficiency,[81] and some researchers recommend screening or prevention strategies.[82]
Lactic acidosis
Lactic acidosis almost never occurs with metformin exposure during routine medical care.[83] Rates of metformin-associated lactic acidosis are about nine per 100,000 persons/year, which is similar to the background rate of lactic acidosis in the general population.[84] A systematic review concluded no data exists to definitively link metformin to lactic acidosis.[85]
Metformin is generally safe in people with mild to moderate chronic kidney disease, with proportional reduction of metformin dose according to severity of estimated glomerular filtration rate (eGFR) and with periodic assessment of kidney function, (e.g., periodic plasma creatinine measurement).[86] The US Food and Drug Administration (FDA) recommends avoiding the use of metformin in more severe chronic kidney disease, below the eGFR cutoff of 30 mL/minute/1.73 m2.[87] Lactate uptake by the liver is diminished with metformin use because lactate is a substrate for hepatic gluconeogenesis, a process that metformin inhibits. In healthy individuals, this slight excess is cleared by other mechanisms (including uptake by unimpaired kidneys), and no significant elevation in blood levels of lactate occurs.[39] Given severely impaired kidney function, clearance of metformin and lactate is reduced, increasing levels of both, and possibly causing lactic acid buildup. Because metformin decreases liver uptake of lactate, any condition that may precipitate lactic acidosis is a contraindication. Common causes include alcoholism (due to depletion of NAD+ stores), heart failure, and respiratory disease (due to inadequate tissue oxygenation); the most common cause is kidney disease.[88]
Metformin-associated lactate production may also take place in the large intestine, which could potentially contribute to lactic acidosis in those with risk factors.[89] The clinical significance of this is unknown, though, and the risk of metformin-associated lactic acidosis is most commonly attributed to decreased hepatic uptake rather than increased intestinal production.[39][88][90]
Overdose
The most common symptoms following an overdose include vomiting, diarrhea, abdominal pain, tachycardia, drowsiness, and rarely, hypoglycemia or hyperglycemia.[91][92] Treatment of metformin overdose is generally supportive, as no specific antidote is known. Extracorporeal treatments are recommended in severe overdoses.[93] Due to metformin's low molecular weight and lack of plasma protein binding, these techniques have the benefit of removing metformin from the blood plasma, preventing further lactate overproduction.[93]
Metformin may be quantified in blood, plasma, or serum to monitor therapy, confirm a diagnosis of poisoning, or to assist in a forensic death investigation. Blood or plasma metformin concentrations are usually in a range of 1–4 mg/L in persons receiving therapeutic doses, 40–120 mg/L in victims of acute overdosage, and 80–200 mg/L in fatalities. Chromatographic techniques are commonly employed.[94][95]
The risk of metformin-associated lactic acidosis is also increased by a massive overdose of metformin, although even quite large doses are often not fatal.[96]
Interactions
The H2-receptor antagonist cimetidine causes an increase in the plasma concentration of metformin by reducing clearance of metformin by the kidneys;[97] both metformin and cimetidine are cleared from the body by tubular secretion, and both, particularly the cationic (positively charged) form of cimetidine, may compete for the same transport mechanism.[7] A small double-blind, randomized study found the antibiotic cephalexin to also increase metformin concentrations by a similar mechanism;[98] theoretically, other cationic medications may produce the same effect.[7]
Metformin also interacts with anticholinergic medications, due to their effect on gastric motility. Anticholinergic drugs reduce gastric motility, prolonging the time drugs spend in the gastrointestinal tract. This impairment may lead to more metformin being absorbed than without the presence of an anticholinergic drug, thereby increasing the concentration of metformin in the plasma and increasing the risk for adverse effects.[99]
Pharmacology
Mechanism of action
The molecular mechanism of metformin is not completely understood. Multiple potential mechanisms of action have been proposed: inhibition of the mitochondrial respiratory chain (complex I), activation of AMP-activated protein kinase (AMPK), inhibition of glucagon-induced elevation of cyclic adenosine monophosphate (cAMP) with reduced activation of protein kinase A (PKA), complex IV–mediated inhibition of the GPD2 variant of mitochondrial glycerol-3-phosphate dehydrogenase (thereby reducing glycerol-derived hepatic gluconeogenesis), and an effect on gut microbiota.[23][100][101][102]
Metformin exerts an anorexiant effect in most people, decreasing caloric intake.[22] Metformin decreases gluconeogenesis (glucose production) in the liver.[89][17] Metformin inhibits basal secretion from the pituitary gland of growth hormone, adrenocorticotropic hormone, follicle stimulating hormone, and expression of proopiomelanocortin,[103] which in part accounts for its insulin-sensitizing effect with multiple actions on tissues including the liver, skeletal muscle, endothelium, adipose tissue, and the ovaries.[53][29] The average patient with type 2 diabetes has three times the normal rate of gluconeogenesis; metformin treatment reduces this by over one-third.[104]
Activation of AMPK was required for metformin's inhibitory effect on liver glucose production.[105] AMPK is an enzyme that plays an important role in insulin signalling, whole-body energy balance, and the metabolism of glucose and fats.[106] AMPK activation is required for an increase in the expression of small heterodimer partner, which in turn inhibited the expression of the hepatic gluconeogenic genes phosphoenolpyruvate carboxykinase and glucose 6-phosphatase.[107] Metformin is frequently used in research along with AICA ribonucleotide as an AMPK agonist. The mechanism by which biguanides increase the activity of AMPK remains uncertain: metformin increases the concentration of cytosolic adenosine monophosphate (AMP) (as opposed to a change in total AMP or total AMP/adenosine triphosphate) which could activate AMPK allosterically at high levels;[108] a newer theory involves binding to PEN-2.[109] Metformin inhibits cyclic AMP production, blocking the action of glucagon, and thereby reducing fasting glucose levels.[110] Metformin also induces a profound shift in the faecal microbial community profile in diabetic mice, and this may contribute to its mode of action possibly through an effect on glucagon-like peptide-1 secretion.[101]
In addition to suppressing hepatic glucose production, metformin increases insulin sensitivity, enhances peripheral glucose uptake (by inducing the phosphorylation of GLUT4 enhancer factor), decreases insulin-induced suppression of fatty acid oxidation,[111] and decreases the absorption of glucose from the gastrointestinal tract. Increased peripheral use of glucose may be due to improved insulin binding to insulin receptors.[112] The increase in insulin binding after metformin treatment has also been demonstrated in patients with type 2 diabetes.[113]
AMPK probably also plays a role in increased peripheral insulin sensitivity, as metformin administration increases AMPK activity in skeletal muscle.[114] AMPK is known to cause GLUT4 deployment to the plasma membrane, resulting in insulin-independent glucose uptake.[115] Some metabolic actions of metformin do appear to occur by AMPK-independent mechanisms, however AMPK likely has a modest overall effect and its activity is not likely to directly decrease gluconeogenesis in the liver.[116]
Metformin has indirect antiandrogenic effects in women with insulin resistance, such as those with PCOS, due to its beneficial effects on insulin sensitivity.[117] It may reduce testosterone levels in such women by as much as 50%.[117] A Cochrane review, though, found that metformin was only slightly effective for decreasing androgen levels in women with PCOS.[118]
Metformin also has significant effects on the gut microbiome, such as its effect on increasing agmatine production by gut bacteria, but the relative importance of this mechanism compared to other mechanisms is uncertain.[119][120][121]
Due to its effect on GLUT4 and AMPK, metformin has been described as an exercise mimetic.[122][123]
Pharmacokinetics
Metformin has an oral bioavailability of 50–60% under fasting conditions, and is absorbed slowly.[7][124] Peak plasma concentrations (Cmax) are reached within 1–3 hours of taking immediate-release metformin and 4–8 hours with extended-release formulations.[7][124] The plasma protein binding of metformin is negligible, as reflected by its very high apparent volume of distribution (300–1000 L after a single dose). Steady state is usually reached in 1–2 days.[7]
Metformin has acid dissociation constant values (pKa) of 2.8 and 11.5, so it exists very largely as the hydrophilic cationic species at physiological pH values. The metformin pKa values make it a stronger base than most other basic medications with less than 0.01% nonionized in blood. Furthermore, the lipid solubility of the nonionized species is slight as shown by its low logP value (log(10) of the distribution coefficient of the nonionized form between octanol and water) of −1.43. These chemical parameters indicate low lipophilicity and, consequently, rapid passive diffusion of metformin through cell membranes is unlikely. As a result of its low lipid solubility it requires the transporter SLC22A1 in order for it to enter cells.[125][126] The logP of metformin is less than that of phenformin (−0.84) because two methyl substituents on metformin impart lesser lipophilicity than the larger phenylethyl side chain in phenformin. More lipophilic derivatives of metformin are presently under investigation with the aim of producing prodrugs with superior oral absorption than metformin.[127]
Metformin is not metabolized. It is cleared from the body by tubular secretion and excreted unchanged in the urine; it is undetectable in blood plasma within 24 hours of a single oral dose.[7][128] The average elimination half-life in plasma is 6.2 hours.[7] Metformin is distributed to (and appears to accumulate in) red blood cells, with a much longer elimination half-life: 17.6 hours[7] (reported as ranging from 18.5 to 31.5 hours in a single-dose study of nondiabetics).[128]
Some evidence indicates that liver concentrations of metformin in humans may be two to three times higher than plasma concentrations, due to portal vein absorption and first-pass uptake by the liver in oral administration.[116]
Chemistry
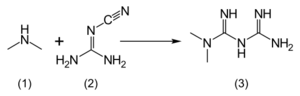
Metformin hydrochloride (1,1-dimethylbiguanide hydrochloride) is freely soluble in water, slightly soluble in ethanol, but almost insoluble in acetone, ether, or chloroform. The pKa of metformin is 12.4.[129] The usual synthesis of metformin, originally described in 1922, involves the one-pot reaction of dimethylamine hydrochloride and 2-cyanoguanidine over heat.[130][131]
According to the procedure described in the 1975 Aron patent,[132] and the Pharmaceutical Manufacturing Encyclopedia,[133] equimolar amounts of dimethylamine and 2-cyanoguanidine are dissolved in toluene with cooling to make a concentrated solution, and an equimolar amount of hydrogen chloride is slowly added. The mixture begins to boil on its own, and after cooling, metformin hydrochloride precipitates with a 96% yield.
Derivatives
A new derivative HL156A, also known as IM156, is a potential new drug for medical use.[134][135][136][137][138][139]
History
The biguanide class of antidiabetic medications, which also includes the withdrawn agents phenformin and buformin, originates from the French lilac or goat's rue (Galega officinalis), a plant used in folk medicine for several centuries.[140] G. officinalis itself does not contain any of these medications, but isoamylene guanidine; phenformin, buformin, and metformin are chemically synthesized compounds composed of two guanidine molecules, and are more lipophilic than the plant-derived parent compound.[140]
Metformin was first described in the scientific literature in 1922, by Emil Werner and James Bell, as a product in the synthesis of N,N-dimethylguanidine.[130] In 1929, Slotta and Tschesche discovered its sugar-lowering action in rabbits, finding it the most potent biguanide analog they studied.[141] This result was ignored, as other guanidine analogs such as the synthalins, took over and were themselves soon overshadowed by insulin.[142]
Interest in metformin resumed at the end of the 1940s. In 1950, metformin, unlike some other similar compounds, was found not to decrease blood pressure and heart rate in animals.[143] That year, Filipino physician Eusebio Y. Garcia[144] used metformin (he named it Fluamine) to treat influenza; he noted the medication "lowered the blood sugar to minimum physiological limit" and was not toxic. Garcia believed metformin to have bacteriostatic, antiviral, antimalarial, antipyretic, and analgesic actions.[145] In a series of articles in 1954, Polish pharmacologist Janusz Supniewski[146] was unable to confirm most of these effects, including lowered blood sugar. Instead he observed antiviral effects in humans.[147][148]
French diabetologist Jean Sterne studied the antihyperglycemic properties of galegine, an alkaloid isolated from G. officinalis, which is related in structure to metformin, and had seen brief use as an antidiabetic before the synthalins were developed.[149] Later, working at Laboratoires Aron in Paris, he was prompted by Garcia's report to reinvestigate the blood sugar-lowering activity of metformin and several biguanide analogs. Sterne was the first to try metformin on humans for the treatment of diabetes; he coined the name "Glucophage" (glucose eater) for the medication and published his results in 1957.[142][149]
Metformin became available in the British National Formulary in 1958. It was sold in the UK by a small Aron subsidiary called Rona.[150]
Broad interest in metformin was not rekindled until the withdrawal of the other biguanides in the 1970s.[4] Metformin was approved in Canada in 1972,[4] but did not receive approval by the U.S. Food and Drug Administration (FDA) for type 2 diabetes until 1994.[151] Produced under license by Bristol-Myers Squibb, Glucophage was the first branded formulation of metformin to be marketed in the U.S., beginning on 3 March 1995.[152] Generic formulations are available in several countries, and metformin is believed to have become the world's most widely prescribed antidiabetic medication.[149]
Society and culture
Environmental
Metformin and its major transformation product guanylurea are present in wastewater treatment plant effluents and regularly detected in surface waters. Guanylurea concentrations above 200 μg/L have been measured in the German river Erpe, which are amongst the highest reported for pharmaceutical transformation products in aquatic environments.[153]
Formulations
The name "Metformin" is the BAN, USAN, and INN for this medication, and is sold under several trade names. Common brand names include Glucophage, Riomet, Fortamet, and Glumetza in the US.[154] In other areas of the world, there is also Obimet, Gluformin, Dianben, Diabex, Diaformin, Metsol, Siofor, Metfogamma and Glifor.[155][156] There are several formulations of Metformin available to the market, and all but the liquid form have generic equivalents.[154] Metformin IR (immediate release) is available in 500-, 850-, and 1000-mg tablets, while Metformin XR (extended release) is available in 500-, 750-, and 1000-mg strengths (also sold as Fortamet, Glumetza, and Glucophage XR in the US). Also available is liquid metformin (sold as Riomet in the US), where 5 mL of solution contains the same amount of drug as a 500-mg tablet.
Combination with other medications
When used for type 2 diabetes, metformin is often prescribed in combination with other medications.
Several are available as fixed-dose combinations, with the potential to reduce pill burden, decrease cost, and simplify administration.[157][158]
Thiazolidinediones (glitazones)
Rosiglitazone
A combination of metformin and rosiglitazone was released in 2002, and sold as Avandamet by GlaxoSmithKline,[159] or as a generic medication.[160] Formulations are 500/1, 500/2, 500/4, 1000/2, and 1000 mg/4 mg of metformin/rosiglitazone.
By 2009, it had become the most popular metformin combination.[161]
In 2005, the stock of Avandamet was removed from the market, after inspections showed the factory where it was produced was violating good manufacturing practices.[162] The medication pair continued to be prescribed separately, and Avandamet was again available by the end of that year. A generic formulation of metformin/rosiglitazone from Teva received tentative approval from the FDA and reached the market in early 2012.[163]
However, following a meta-analysis in 2007 that linked the medication's use to an increased risk of heart attack,[164] concerns were raised over the safety of medicines containing rosiglitazone. In September 2010, the European Medicines Agency recommended that the medication be suspended from the European market because the benefits of rosiglitazone no longer outweighed the risks.[165][166]
It was withdrawn from the market in the UK and India in 2010,[167] and in New Zealand and South Africa in 2011.[168] From November 2011 until November 2013 the FDA[169] did not allow rosiglitazone or metformin/rosiglitazone to be sold without a prescription; moreover, makers were required to notify patients of the risks associated with its use, and the drug had to be purchased by mail order through specified pharmacies.[170][171]
In November 2013, the FDA lifted its earlier restrictions on rosiglitazone after reviewing the results of the 2009 RECORD clinical trial (a six-year, open-label randomized control trial), which failed to show elevated risk of heart attack or death associated with the medication.[172][173][174]
Pioglitazone
The combination of metformin and pioglitazone (Actoplus Met, Piomet, Politor, Glubrava) is available in the US and the European Union.[175][176][177][178][179]
DPP-4 inhibitors
Dipeptidyl peptidase-4 inhibitors inhibit dipeptidyl peptidase-4 and thus reduce glucagon and blood glucose levels.
DPP-4 inhibitors combined with metformin include a sitagliptin/metformin combination (Janumet),[180][181] a saxagliptin/metformin combination (Kombiglyze XR, Komboglyze),[182][183] and an alogliptin/metformin combination (Kazano, Vipdomet).[184][185]
Linagliptin combined with metformin hydrochloride is sold under the brand name Jentadueto.[186][187][188] As of August 2021, linagliptin/metformin is available as a generic medicine in the US.[189]
SGLT-2 inhibitors
There are combinations of metformin with the SGLT-2 inhibitors dapagliflozin, empagliflozin, and canagliflozin.
Sulfonylureas
Sulfonylureas act by increasing insulin release from the beta cells in the pancreas.[190]
A 2019 systematic review suggested that there is limited evidence if the combined used of metformin with sulfonylurea compared to the combination of metformin plus another glucose-lowering intervention, provides benefit or harm in mortality, severe adverse events, macrovascular and microvascular complications.[191] Combined metformin and sulfonylurea therapy did appear to lead to higher risk of hypoglicaemia.[191]
Metformin is available combined with the sulfonylureas glipizide (Metaglip) and glibenclamide (US: glyburide) (Glucovance). Generic formulations of metformin/glipizide and metformin/glibenclamide are available (the latter is more popular).[192]
Meglitinide
Meglitinides are similar to sulfonylureas, as they bind to beta cells in the pancreas, but differ by the site of binding to the intended receptor and the drugs' affinities to the receptor.[190] As a result, they have a shorter duration of action compared to sulfonylureas, and require higher blood glucose levels to begin to secrete insulin. Both meglitinides, known as nateglinide and repanglinide, is sold in formulations combined with metformin. A repaglinide/metformin combination is sold as Prandimet, or as its generic equivalent.[193][194]
Triple combination
The combination of metformin with dapagliflozen and saxagliptin is available in the United States as Qternmet XR.[195][196]
The combination of metformin with pioglitazone and glibenclamide[197] is available in India as Accuglim-MP, Adglim MP, and Alnamet-GP, along with the Philippines as Tri-Senza.[156]
The combination of metformin with pioglitazone and lipoic acid is available in Turkey as Pional.[156]
Impurities
In December 2019, the US FDA announced that it learned that some metformin medicines manufactured outside the United States might contain a nitrosamine impurity called N-nitrosodimethylamine (NDMA), classified as a probable human carcinogen, at low levels.[198] Health Canada announced that it was assessing NDMA levels in metformin.[199]
In February 2020, the FDA found NDMA levels in some tested metformin samples that did not exceed the acceptable daily intake.[200][201]
In February 2020, Health Canada announced a recall of Apotex immediate-release metformin,[202] followed in March by recalls of Ranbaxy metformin[203] and in March by Jamp metformin.[204]
In May 2020, the FDA asked five companies to voluntarily recall their sustained-release metformin products.[205][206][207][208][209][210] The five companies were not named, but they were revealed to be Amneal Pharmaceuticals, Actavis Pharma, Apotex Corp, Lupin Pharma, and Marksans Pharma Limited in a letter sent to Valisure, the pharmacy that had first alerted the FDA to this contaminant in metformin via a Citizen Petition.[211]
In June 2020, the FDA posted its laboratory results showing NDMA amounts in metformin products it tested.[212] It found NDMA in certain lots of ER metformin, and is recommending companies recall lots with levels of NDMA above the acceptable intake limit of 96 nanograms per day.[212] The FDA is also collaborating with international regulators to share testing results for metformin.[212]
In July 2020, Lupin Pharmaceuticals pulled all lots (batches) of metformin after discovering unacceptably high levels of NDMA in tested samples.[213]
In August 2020, Bayshore Pharmaceuticals recalled two lots of tablets.[214]
Research
Metformin has been studied for its effects on multiple other conditions, including:
- Non-alcoholic fatty liver disease[215][216][217]
- Premature puberty[218][219][220]
- Cancer[155][221][222]
- Cardiovascular disease in people with diabetes[223]
- Aging[224][69]
While metformin may reduce body weight in persons with fragile X syndrome, whether it improves neurological or psychiatric symptoms is uncertain.[225] Metformin has been studied in vivo (C. elegans and crickets) for effects on aging.[126][226] A 2017 review found that people with diabetes who were taking metformin had lower all-cause mortality. They also had reduced cancer and cardiovascular disease compared with those on other therapies.[223]
There is also some research suggesting that although metformin prevents diabetes, it does not reduce the risk of cancer and cardiovascular disease and thus does not extend lifespan in non-diabetic individuals.[227] Furthermore, some studies suggest that long-term chronic use of metformin by healthy individuals may develop vitamin B12 deficiency.[228]
References
- ↑ "Disposition of metformin (N,N-dimethylbiguanide) in man". Clinical Pharmacology and Therapeutics 24 (6): 683–93. December 1978. doi:10.1002/cpt1978246683. PMID 710026.
- ↑ "Metformin Use During Pregnancy". 10 September 2019. https://www.drugs.com/pregnancy/metformin.html.
- ↑ "Metformin SANDOZ metformin hydrochloride 850mg tablet bottle (148270)". 27 May 2022. https://www.tga.gov.au/resources/artg/148270.
- ↑ 4.0 4.1 4.2 "The status of metformin in Canada". Canadian Medical Association Journal 128 (1): 24–6. January 1983. PMID 6847752.
- ↑ "Metformin Hydrochloride". Health Canada. https://health-products.canada.ca/dpd-bdpp/info?lang=eng&code=17104.
- ↑ "Glucophage 500 mg film coated tablets - Summary of Product Characteristics (SmPC)". 25 October 2022. https://www.medicines.org.uk/emc/product/987/smpc.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 "Glucophage (metformin hydrochloride) tablets, for oral use; Glucophage XR (metformin hydrochloride) extended-release tablets, for oral use Initial U.S. Approval:1995". https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=453104.
- ↑ 8.0 8.1 8.2 8.3 8.4 "Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus". Drugs 49 (5): 721–49. May 1995. doi:10.2165/00003495-199549050-00007. PMID 7601013.
- ↑ "Metformin: new understandings, new uses". Drugs 63 (18): 1879–94. 2003. doi:10.2165/00003495-200363180-00001. PMID 12930161.
- ↑ "Metformin". https://www.chemsrc.com/en/cas/657-24-9_889579.html.
- ↑ "9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022". Diabetes Care 45 (Suppl 1): S125–S143. January 2022. doi:10.2337/dc22-s009. PMID 34964831.
- ↑ 12.0 12.1 "2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD". European Heart Journal 41 (2): 255–323. January 2020. doi:10.1093/eurheartj/ehz486. PMID 31497854.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 "Metformin Hydrochloride". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/metformin-hydrochloride.html.
- ↑ "Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis". Annals of Internal Medicine 164 (11): 740–751. June 2016. doi:10.7326/M15-2650. PMID 27088241.
- ↑ "Metformin in prevention and treatment of antipsychotic-induced weight gain: a systematic review and meta-analysis". BMC Psychiatry 16 (1): 341. October 2016. doi:10.1186/s12888-016-1049-5. PMID 27716110.
- ↑ "Type 2 diabetes and metformin. First choice for monotherapy: weak evidence of efficacy but well-known and acceptable adverse effects". Prescrire International 23 (154): 269–272. November 2014. PMID 25954799.
- ↑ 17.0 17.1 "Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium". Acta Physiologica 219 (1): 138–151. January 2017. doi:10.1111/apha.12644. PMID 26680745.
- ↑ "Do Patients Die with or from Metformin-Associated Lactic Acidosis (MALA)? Systematic Review and Meta-analysis of pH and Lactate as Predictors of Mortality in MALA". Journal of Medical Toxicology 16 (2): 222–229. April 2020. doi:10.1007/s13181-019-00755-6. PMID 31907741.
- ↑ "Use of metformin in the setting of mild-to-moderate renal insufficiency". Diabetes Care 34 (6): 1431–7. June 2011. doi:10.2337/dc10-2361. PMID 21617112.
- ↑ 20.0 20.1 "GDF15 mediates the effects of metformin on body weight and energy balance". Nature 578 (7795): 444–448. February 2020. doi:10.1038/s41586-019-1911-y. PMID 31875646.
- ↑ "Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss". Nature Metabolism 1 (12): 1202–1208. December 2019. doi:10.1038/s42255-019-0146-4. PMID 32694673.
- ↑ 22.0 22.1 "Medical Management of Diabesity: Do We Have Realistic Targets?". Current Diabetes Reports 17 (1): 4. January 2017. doi:10.1007/s11892-017-0828-9. PMID 28101792.
- ↑ 23.0 23.1 "Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis". Proceedings of the National Academy of Sciences of the United States of America 119 (10): e2122287119. March 2022. doi:10.1073/pnas.2122287119. PMID 35238637. Bibcode: 2022PNAS..11922287L.
- ↑ 24.0 24.1 24.2 Analogue-based Drug Discovery II. John Wiley & Sons. 2010. pp. 49. ISBN 978-3-527-63212-1. https://books.google.com/books?id=h2Kd8ci4Ln8C&pg=PA49.
- ↑ Herb, nutrient, and drug interactions : clinical implications and therapeutic strategies. St. Louis, Mo.: Mosby/Elsevier. 2008. p. 217. ISBN 978-0-323-02964-3. https://books.google.com/books?id=49kLK--eumEC&pg=PA217.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Metformin - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Metformin.
- ↑ 29.0 29.1 "Metformin in polycystic ovary syndrome: systematic review and meta-analysis". BMJ 327 (7421): 951–3. October 2003. doi:10.1136/bmj.327.7421.951. PMID 14576245.
- ↑ "Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations". Annals of Internal Medicine 154 (9): 602–13. May 2011. doi:10.7326/0003-4819-154-9-201105030-00336. PMID 21403054.
- ↑ 31.0 31.1 "Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)". Diabetes Care 35 (6): 1364–79. June 2012. doi:10.2337/dc12-0413. PMID 22517736.
- ↑ "Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians". Annals of Internal Medicine 156 (3): 218–31. February 2012. doi:10.7326/0003-4819-156-3-201202070-00011. PMID 22312141.
- ↑ "Is metformin still the most efficacious first-line oral hypoglycaemic drug in treating type 2 diabetes? A network meta-analysis of randomized controlled trials". Obesity Reviews 20 (1): 1–12. January 2019. doi:10.1111/obr.12753. PMID 30230172.
- ↑ "ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD - summary". Diabetes & Vascular Disease Research 11 (3): 133–73. May 2014. doi:10.1177/1479164114525548. PMID 24800783.
- ↑ "Metformin monotherapy for adults with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews 2020 (6): CD012906. June 2020. doi:10.1002/14651858.CD012906.pub2. PMID 32501595.
- ↑ 36.0 36.1 "Efficacy of metformin in the treatment of NIDDM. Meta-analysis". Diabetes Care 22 (1): 33–7. January 1999. doi:10.2337/diacare.22.1.33. PMID 10333900.
- ↑ "Metformin and body weight". International Journal of Obesity 32 (1): 61–72. January 2008. doi:10.1038/sj.ijo.0803695. PMID 17653063.
- ↑ "Drug interventions for the treatment of obesity in children and adolescents". The Cochrane Database of Systematic Reviews 11 (11): CD012436. November 2016. doi:10.1002/14651858.CD012436. PMID 27899001.
- ↑ 39.0 39.1 39.2 39.3 "Chapter 27: Diabetes Mellitus & Hypoglycemia". Current Medical Diagnosis and Treatment 2010 (49th ed.). McGraw-Hill Medical. 2009. pp. 1092–93. ISBN 978-0-07-162444-2. https://archive.org/details/isbn_9780071624442/page/1092.
- ↑ 40.0 40.1 40.2 "Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus". Annals of Internal Medicine 147 (6): 386–99. September 2007. doi:10.7326/0003-4819-147-6-200709180-00178. PMID 17638715.
- ↑ Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill. 2005. ISBN 978-0-07-141613-9.
- ↑ "Glucophage package insert". Princeton, NJ: Bristol-Myers Squibb Company. 2009. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18054.
- ↑ 43.0 43.1 43.2 43.3 "Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews 2019 (12): CD008558. December 2019. doi:10.1002/14651858.CD008558.pub2. PMID 31794067.
- ↑ 44.0 44.1 "Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility". The Cochrane Database of Systematic Reviews 11 (2): CD003053. November 2017. doi:10.1002/14651858.CD003053.pub6. PMID 29183107. "Our updated review suggests that metformin alone may be beneficial over placebo for live birth, although the evidence quality was low.".
- ↑ "Pregnancy outcomes and the effect of metformin treatment in women with polycystic ovary syndrome: an overview". Acta Obstetricia et Gynecologica Scandinavica 91 (6): 658–78. June 2012. doi:10.1111/j.1600-0412.2012.01385.x. PMID 22375613.
- ↑ "Effects of metformin use in pregnant patients with polycystic ovary syndrome". Journal of Human Reproductive Sciences 5 (2): 166–9. May 2012. doi:10.4103/0974-1208.101012. PMID 23162354.
- ↑ "Pharmacological and surgical treatment of nonreproductive outcomes in polycystic ovary syndrome: An overview of systematic reviews". Clinical Endocrinology 89 (5): 535–553. November 2018. doi:10.1111/cen.13753. PMID 29846959.
- ↑ 48.0 48.1 48.2 48.3 48.4 "Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome". The Cochrane Database of Systematic Reviews 2020 (12): CD006105. December 2020. doi:10.1002/14651858.CD006105.pub4. PMID 33347618.
- ↑ "Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes". The Cochrane Database of Systematic Reviews 2018 (7): CD010564. July 2018. doi:10.1002/14651858.CD010564.pub2. PMID 30039871.
- ↑ National Collaborating Centre for Women's and Children's Health (2004). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians and Gynaecologists. pp. 58–59. ISBN 978-1-900364-97-3. http://www.nice.org.uk/nicemedia/pdf/cg011fullguideline.pdf.
- ↑ "Metformin therapy for the management of infertility in women with polycystic ovary syndrome". Scientific Advisory Committee Opinion Paper 13. Royal College of Obstetricians and Gynaecologists. December 2008. http://www.rcog.org.uk/files/rcog-corp/uploaded-files/SAC13metformin-minorrevision.pdf.
- ↑ The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (March 2008). "Consensus on infertility treatment related to polycystic ovary syndrome". Human Reproduction 23 (3): 462–77. doi:10.1093/humrep/dem426. PMID 18308833.
- ↑ 53.0 53.1 "Metformin in polycystic ovary syndrome". Annals of the New York Academy of Sciences 1205 (1): 192–8. September 2010. doi:10.1111/j.1749-6632.2010.05679.x. PMID 20840272. Bibcode: 2010NYASA1205..192D.
- ↑ "Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome". European Journal of Endocrinology 162 (2): 193–212. February 2010. doi:10.1530/EJE-09-0733. PMID 19841045.
- ↑ "Preventative and Therapeutic Effects of Metformin in Gastric Cancer: A New Contribution of an Old Friend" (in English). Cancer Management and Research 12: 8545–8554. 16 September 2020. doi:10.2147/CMAR.S264032. PMID 32982447.
- ↑ "The Role of Metformin in Gastric Cancer Treatment" (in en-US). 29 October 2023. https://thewitfire.in/2023/10/29/the-role-of-metformin-in-gastric-cancer-treatment/.
- ↑ "Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis". Diabetic Medicine 34 (1): 27–36. January 2017. doi:10.1111/dme.13150. PMID 27150509.
- ↑ "Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review". Obstetrics and Gynecology 113 (1): 193–205. January 2009. doi:10.1097/AOG.0b013e318190a459. PMID 19104375.
- ↑ "Metformin for the treatment of gestational diabetes: An updated meta-analysis". Diabetes Research and Clinical Practice 109 (3): 521–32. September 2015. doi:10.1016/j.diabres.2015.05.017. PMID 26117686.
- ↑ 60.0 60.1 "Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis". BMJ 350: h102. January 2015. doi:10.1136/bmj.h102. PMID 25609400.
- ↑ "Risk of pre-eclampsia in women taking metformin: a systematic review and meta-analysis". Diabetic Medicine 35 (2): 160–172. February 2018. doi:10.1111/dme.13523. PMID 29044702. https://pure.qub.ac.uk/en/publications/8020376c-6e9c-4795-af4b-15441b5773bf. Retrieved 7 April 2023.
- ↑ "Metformin in reproductive health, pregnancy and gynaecological cancer: established and emerging indications". Human Reproduction Update 20 (6): 853–68. 2014. doi:10.1093/humupd/dmu037. PMID 25013215.
- ↑ "Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis". PLOS Medicine 16 (8): e1002848. August 2019. doi:10.1371/journal.pmed.1002848. PMID 31386659.
- ↑ "Metformin: Mechanisms in Human Obesity and Weight Loss". Current Obesity Reports 8 (2): 156–164. June 2019. doi:10.1007/s13679-019-00335-3. PMID 30874963.
- ↑ "Efficacy of adjunctive treatments added to olanzapine or clozapine for weight control in patients with schizophrenia: a systematic review and meta-analysis". TheScientificWorldJournal 2015: 970730. 2015. doi:10.1155/2015/970730. PMID 25664341.
- ↑ "Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis". British Journal of Clinical Pharmacology 71 (3): 377–82. March 2011. doi:10.1111/j.1365-2125.2010.03783.x. PMID 21284696.
- ↑ "Metformin for Clozapine Associated Obesity: A Systematic Review and Meta-Analysis". PLOS ONE 11 (6): e0156208. 2016. doi:10.1371/journal.pone.0156208. PMID 27304831. Bibcode: 2016PLoSO..1156208S.
- ↑ "The use of metformin in type 1 diabetes: a systematic review of efficacy". Diabetologia 53 (5): 809–20. May 2010. doi:10.1007/s00125-009-1636-9. PMID 20057994.
- ↑ 69.0 69.1 "Metformin: A Hopeful Promise in Aging Research". Cold Spring Harbor Perspectives in Medicine 6 (3): a025932. March 2016. doi:10.1101/cshperspect.a025932. PMID 26931809.
- ↑ "Benefits of Metformin in Attenuating the Hallmarks of Aging". Cell Metabolism 32 (1): 15–30. July 2020. doi:10.1016/j.cmet.2020.04.001. PMID 32333835.
- ↑ "Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis". Journal of Alzheimer's Disease 65 (4): 1225–1236. 2018. doi:10.3233/jad-180263. PMID 30149446.
- ↑ "Metformin and Alzheimer's disease, dementia and cognitive impairment: a systematic review protocol". JBI Database of Systematic Reviews and Implementation Reports 15 (8): 2055–2059. August 2017. doi:10.11124/JBISRIR-2017-003380. PMID 28800055.
- ↑ 73.0 73.1 73.2 "Metformin: medicine to treat type 2 diabetes". 25 February 2019. https://www.nhs.uk/medicines/metformin/.
- ↑ 74.0 74.1 74.2 74.3 "METFORMIN HYDROCHLORIDE". https://bnf.nice.org.uk/drug/metformin-hydrochloride.html.
- ↑ "Metformin: safety in cardiac patients". Heart 96 (2): 99–102. January 2010. doi:10.1136/hrt.2009.173773. PMID 19564648.
- ↑ "Metformin in chronic kidney disease: time for a rethink". Peritoneal Dialysis International 34 (4): 353–7. June 2014. doi:10.3747/pdi.2013.00344. PMID 24711640.
- ↑ 77.0 77.1 "Metformin: New Preparations and Nonglycemic Benefits". Current Diabetes Reports 17 (1): 5. January 2017. doi:10.1007/s11892-017-0829-8. PMID 28116648.
- ↑ "Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial". Journal of Internal Medicine 254 (5): 455–63. November 2003. doi:10.1046/j.1365-2796.2003.01213.x. PMID 14535967.
- ↑ "Metformin-associated vitamin B12 deficiency". Archives of Internal Medicine 162 (19): 2251–2. October 2002. PMID 12390080. https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/213629.
- ↑ "Metformin and vitamin B12 deficiency". Archives of Internal Medicine 162 (4): 484–5. February 2002. PMID 11863489. https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/211187.
- ↑ "Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial". BMJ 340: c2181. May 2010. doi:10.1136/bmj.c2181. PMID 20488910.
- ↑ "Risk factors of vitamin B(12) deficiency in patients receiving metformin". Archives of Internal Medicine 166 (18): 1975–9. October 2006. doi:10.1001/archinte.166.18.1975. PMID 17030830.
- ↑ "Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes". Diabetologia 52 (1): 17–30. January 2009. doi:10.1007/s00125-008-1157-y. PMID 18941734.
- ↑ "Incidence of lactic acidosis in metformin users". Diabetes Care 22 (6): 925–7. June 1999. doi:10.2337/diacare.22.6.925. PMID 10372243.
- ↑ "Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis". Archives of Internal Medicine 163 (21): 2594–602. November 2003. doi:10.1001/archinte.163.21.2594. PMID 14638559.
- ↑ "Metformin in patients with type 2 diabetes and kidney disease: a systematic review". JAMA 312 (24): 2668–75. 2014. doi:10.1001/jama.2014.15298. PMID 25536258.
- ↑ "FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function". 14 November 2017. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain.
- ↑ 88.0 88.1 "Chapter 29: Pharmacology of the Endocrine Pancreas". Principles of pharmacology: the pathophysiologic basis of drug therapy. Philadelphia: Lippincott, Williams & Wilkins. 2005. pp. 540–41. ISBN 978-0-7817-4678-6.
- ↑ 89.0 89.1 "Metformin: an update". Annals of Internal Medicine 137 (1): 25–33. July 2002. doi:10.7326/0003-4819-137-1-200207020-00009. PMID 12093242.
- ↑ "Chapter 60: Insulin, Oral Hypoglycemic Agents, and the Pharmacology of the Endocrine Pancreas". Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. 2006. ISBN 978-0-07-142280-2.
- ↑ "Adult metformin ingestions reported to Texas poison control centers, 2000-2006". Human & Experimental Toxicology 27 (7): 575–83. July 2008. doi:10.1177/0960327108090589. PMID 18829734. Bibcode: 2008HETox..27..575F.
- ↑ "Fatal metformin overdose presenting with progressive hyperglycemia". The Western Journal of Emergency Medicine 9 (3): 160–4. August 2008. PMID 19561734.
- ↑ 93.0 93.1 "Extracorporeal Treatment for Metformin Poisoning: Systematic Review and Recommendations From the Extracorporeal Treatments in Poisoning Workgroup". Critical Care Medicine 43 (8): 1716–30. August 2015. doi:10.1097/CCM.0000000000001002. PMID 25860205.
- ↑ "Determination of metformin in human plasma using hydrophilic interaction liquid chromatography-tandem mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 877 (29): 3695–700. November 2009. doi:10.1016/j.jchromb.2009.09.020. PMID 19783231.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 939–940.
- ↑ "The management of metformin overdose". Anaesthesia 53 (7): 698–701. July 1998. doi:10.1046/j.1365-2044.1998.436-az0549.x. PMID 9771180.
- ↑ "Reduction of metformin renal tubular secretion by cimetidine in man". British Journal of Clinical Pharmacology 23 (5): 545–51. May 1987. doi:10.1111/j.1365-2125.1987.tb03090.x. PMID 3593625.
- ↑ "Effect of cephalexin on the pharmacokinetics of metformin in healthy human volunteers". Drug Metabolism and Drug Interactions 19 (1): 41–8. 2002. doi:10.1515/dmdi.2002.19.1.41. PMID 12222753.
- ↑ "Clinically and pharmacologically relevant interactions of antidiabetic drugs". Therapeutic Advances in Endocrinology and Metabolism 7 (2): 69–83. April 2016. doi:10.1177/2042018816638050. PMID 27092232.
- ↑ "Molecular mechanism of action of metformin: old or new insights?". Diabetologia 56 (9): 1898–906. September 2013. doi:10.1007/s00125-013-2991-0. PMID 23835523.
- ↑ 101.0 101.1 "The antidiabetic gutsy role of metformin uncovered?". Gut 63 (5): 706–7. May 2014. doi:10.1136/gutjnl-2013-305370. PMID 23840042.
- ↑ "Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase". Nature 510 (7506): 542–6. June 2014. doi:10.1038/nature13270. PMID 24847880. Bibcode: 2014Natur.510..542M.
- ↑ "The Pituitary Gland is a Novel Major Site of Action of Metformin in Non-Human Primates: a Potential Path to Expand and Integrate Its Metabolic Actions". Cellular Physiology and Biochemistry 49 (4): 1444–1459. 2018. doi:10.1159/000493448. PMID 30205369.
- ↑ "Mechanism by which metformin reduces glucose production in type 2 diabetes". Diabetes 49 (12): 2063–9. December 2000. doi:10.2337/diabetes.49.12.2063. PMID 11118008.
- ↑ "Role of AMP-activated protein kinase in mechanism of metformin action". The Journal of Clinical Investigation 108 (8): 1167–74. October 2001. doi:10.1172/JCI13505. PMID 11602624.
- ↑ "AMP-activated protein kinase in metabolic control and insulin signaling". Circulation Research 100 (3): 328–41. February 2007. doi:10.1161/01.RES.0000256090.42690.05. PMID 17307971.
- ↑ "Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP". Diabetes 57 (2): 306–14. February 2008. doi:10.2337/db07-0381. PMID 17909097.
- ↑ "Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration". American Journal of Physiology. Heart and Circulatory Physiology 293 (1): H457-66. July 2007. doi:10.1152/ajpheart.00002.2007. PMID 17369473.
- ↑ "Low-dose metformin targets the lysosomal AMPK pathway through PEN2". Nature 603 (7899): 159–165. March 2022. doi:10.1038/s41586-022-04431-8. PMID 35197629. Bibcode: 2022Natur.603..159M.
- ↑ "Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP". Nature 494 (7436): 256–60. February 2013. doi:10.1038/nature11808. PMID 23292513. Bibcode: 2013Natur.494..256M.
- ↑ "Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle". American Journal of Physiology. Endocrinology and Metabolism 291 (1): E182-9. July 2006. doi:10.1152/ajpendo.00272.2005. PMID 16478780.
- ↑ "Metformin". The New England Journal of Medicine 334 (9): 574–9. February 1996. doi:10.1056/NEJM199602293340906. PMID 8569826.
- ↑ "Mechanism of action of metformin: insulin receptor and postreceptor effects in vitro and in vivo". The Journal of Clinical Endocrinology and Metabolism 63 (4): 898–905. October 1986. doi:10.1210/jcem-63-4-898. PMID 3745404.
- ↑ "Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes". Diabetes 51 (7): 2074–2081. July 2002. doi:10.2337/diabetes.51.7.2074. PMID 12086935.
- ↑ "AMPK: a nutrient and energy sensor that maintains energy homeostasis". Nature Reviews. Molecular Cell Biology 13 (4): 251–262. March 2012. doi:10.1038/nrm3311. PMID 22436748.
- ↑ 116.0 116.1 "Cellular and Molecular Mechanisms of Metformin Action". Endocrine Reviews 42 (1): 77–96. January 2021. doi:10.1210/endrev/bnaa023. PMID 32897388.
- ↑ 117.0 117.1 "Is There a Role for Antiandrogen Therapy for Hidradenitis Suppurativa? A Systematic Review of Published Data". American Journal of Clinical Dermatology 20 (4): 503–513. August 2019. doi:10.1007/s40257-019-00442-w. PMID 31073704.
- ↑ "Combined oral contraceptives and/or antiandrogens versus insulin sensitizers for polycystic ovary syndrome: a systematic review and meta-analysis". Human Reproduction Update 24 (2): 225–241. March 2018. doi:10.1093/humupd/dmx039. PMID 29293982.
- ↑ "Interaction between drugs and the gut microbiome". Gut 69 (8): 1510–1519. August 2020. doi:10.1136/gutjnl-2019-320204. PMID 32409589.
- ↑ "Bacteria transmit metformin-associated lifespan extension". Nature Reviews. Endocrinology 16 (1): 9–10. January 2020. doi:10.1038/s41574-019-0278-3. PMID 31645681.
- ↑ "Metformin, Microbiome and Protection Against Colorectal Cancer". Digestive Diseases and Sciences 66 (5): 1409–1414. June 2020. doi:10.1007/s10620-020-06390-4. PMID 32533543.
- ↑ "Exercise in a Pill: The Latest on Exercise-Mimetics". Brain Plasticity 2 (2): 153–169. March 2017. doi:10.3233/BPL-160043. PMID 29765854.
- ↑ "Metformin and exercise in type 2 diabetes: examining treatment modality interactions". Diabetes Care 34 (7): 1469–1474. July 2011. doi:10.2337/dc10-2207. PMID 21602430.
- ↑ 124.0 124.1 "Metformin overdose in dogs and cats". Veterinary Medicine (April): 231–33. 2007. http://www.aspca.org/site/DocServer/vetm0407_231-234.pdf?docID=11061.
- ↑ "Metformin: a metabolic disruptor and anti-diabetic drug to target human leukemia". Cancer Letters 346 (2): 188–96. May 2014. doi:10.1016/j.canlet.2014.01.006. PMID 24462823.
- ↑ 126.0 126.1 "Repurposing metformin: an old drug with new tricks in its binding pockets". The Biochemical Journal 471 (3): 307–22. November 2015. doi:10.1042/bj20150497. PMID 26475449.
- ↑ "Clinical pharmacokinetics of metformin". Clinical Pharmacokinetics 50 (2): 81–98. February 2011. doi:10.2165/11534750-000000000-00000. PMID 21241070.
- ↑ 128.0 128.1 "Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects". Diabetes & Metabolism 29 (3): 279–83. June 2003. doi:10.1016/s1262-3636(07)70037-x. PMID 12909816.
- ↑ "Diabetes Drugs: Present and Emerging". Burger's Medicinal Chemistry and Drug Discovery. 2010. pp. 1–38. doi:10.1002/0471266949.bmc198. ISBN 978-0471266945.
- ↑ 130.0 130.1 "The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyandiamide, and methylammonium and dimethylammonium chlorides respectively". J. Chem. Soc., Trans. 121: 1790–95. 1922. doi:10.1039/CT9222101790. https://zenodo.org/record/1860743. Retrieved 4 September 2020.
- ↑ "Hypoglycemic Agents. I Chemical Properties of β-Phenethylbiguanide. A New Hypoglycemic Agent". J Am Chem Soc 81 (9): 2220–25. 1959. doi:10.1021/ja01518a052.
- ↑ "Procédé de préparation de chlorhydrate de diméthylbiguanide" (in fr). Patent FR 2322860. 1975.
- ↑ Pharmaceutical Manufacturing Encyclopedia (Sittig's Pharmaceutical Manufacturing Encyclopedia). 3 (3rd ed.). Norwich, NY: William Andrew. 2007. p. 2208. ISBN 978-0-8155-1526-5.
- ↑ "New metformin derivative HL156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways". Cancer Science 109 (3): 699–709. March 2018. doi:10.1111/cas.13482. PMID 29285837.
- ↑ "HL156A, a novel pharmacological agent with potent adenosine-monophosphate-activated protein kinase (AMPK) activator activity ameliorates renal fibrosis in a rat unilateral ureteral obstruction model". PLOS ONE 13 (8): e0201692. 2018. doi:10.1371/journal.pone.0201692. PMID 30161162. Bibcode: 2018PLoSO..1301692T.
- ↑ "Metformin Derivative HL156A Reverses Multidrug Resistance by Inhibiting HOXC6/ERK1/2 Signaling in Multidrug-Resistant Human Cancer Cells". Pharmaceuticals 13 (9): 218. August 2020. doi:10.3390/ph13090218. PMID 32872293.
- ↑ "Antioxidant modifications induced by the new metformin derivative HL156A regulate metabolic reprogramming in SAMP1/kl (-/-) mice". Aging 10 (9): 2338–2355. September 2018. doi:10.18632/aging.101549. PMID 30222592.
- ↑ "Inhibiting stemness and invasive properties of glioblastoma tumorsphere by combined treatment with temozolomide and a newly designed biguanide (HL156A)". Oncotarget 7 (40): 65643–65659. October 2016. doi:10.18632/oncotarget.11595. PMID 27582539.
- ↑ "HL156A, a novel AMP-activated protein kinase activator, is protective against peritoneal fibrosis in an in vivo and in vitro model of peritoneal fibrosis". American Journal of Physiology. Renal Physiology 310 (5): F342-50. March 2016. doi:10.1152/ajprenal.00204.2015. PMID 26661649.
- ↑ 140.0 140.1 "The blooming of the French lilac". The Journal of Clinical Investigation 108 (8): 1105–7. October 2001. doi:10.1172/JCI14178. PMID 11602616.
- ↑ See Chemical Abstracts, v.23, 42772 (1929) "Über Biguanide, II.: Die blutzucker-senkende Wirkung der Biguanide". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 62 (6): 1398–1405. 1929. doi:10.1002/cber.19290620605.
- ↑ 142.0 142.1 "Metformin – life begins at 50: A symposium held on the occasion of the 43rd Annual Meeting of the European Association for the Study of Diabetes, Amsterdam, the Netherlands, September 2007". The British Journal of Diabetes & Vascular Disease 7 (5): 247–52. September 2007. doi:10.1177/14746514070070051001.
- ↑ "Circulatory and respiratory reflexes caused by aromatic guanidines". British Journal of Pharmacology and Chemotherapy 5 (1): 65–76. March 1950. doi:10.1111/j.1476-5381.1950.tb00578.x. PMID 15405470.
- ↑ About Eusebio Y. Garcia, see: "Search for DOST-NRCP Dr. Eusebio Y. Garcia Award". Philippines Department of Science and Technology. 2005. http://sntpost.stii.dost.gov.ph/frames/aprtojun05/Search_for_DOST_NRCP_13to14.htm.
- ↑ Quoted from Chemical Abstracts, v.45, 24828 (1951) "Flumamine, a new synthetic analgesic and anti-flu drug". Journal of the Philippine Medical Association 26 (7): 287–93. July 1950. PMID 14779282.
- ↑ About Janusz Supniewski, see: "Pharmacology at the Jagiellonian University in Kracow, short review of contribution to global science and cardiovascular research through 400 years of history". Journal of Physiology and Pharmacology 57 (Suppl 1): 119–136. April 2006. PMID 16766803. http://www.jpp.krakow.pl/journal/archive/0406_s1/pdf/119_0406_s1_article.pdf. Retrieved 22 December 2009.
- ↑ See Chemical Abstracts, v. 52, 22272 (1958) "[N-dimethyl-di-guanide and its biological properties]" (in pl). Archivum Immunologiae et Therapiae Experimentalis 2: 1–15. 1954. PMID 13269290.
- ↑ Quoted from Chemical Abstracts, v.49, 74699 (1955) "[Effect of biguanide derivatives on experimental cowpox in rabbits]" (in fr). Bulletin de l'Académie Polonaise des Sciences, Classe 3: Mathématique, Astronomie, Physique, Chimie, Géologie et Géographie 2(Classe II): 161–65. 1954.
- ↑ 149.0 149.1 149.2 "Metformin: its botanical background". Practical Diabetes International 21 (3): 115–17. 2004. doi:10.1002/pdi.606. http://www3.interscience.wiley.com/cgi-bin/fulltext/108564133/HTMLSTART.
- ↑ "Goat's rue - French lilac - Italian fitch - Spanish sainfoin: gallega officinalis and metformin: the Edinburgh connection". The Journal of the Royal College of Physicians of Edinburgh 35 (3): 258–60. October 2005. PMID 16402501. http://www.rcpe.ac.uk/journal/issue/journal_35_3/hadden_goats%20rue.pdf. Retrieved 21 December 2009.
- ↑ "FDA Approves New Diabetes Drug" (Press release). U.S. Food and Drug Administration (FDA). 30 December 1994. Archived from the original on 29 September 2007. Retrieved 6 January 2007.
- ↑ "Drug Approval Package: Glucophage (metformin)". https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/020357Orig1s000rev.pdf.
- ↑ "Determination of polar organic micropollutants in surface and pore water by high-resolution sampling-direct injection-ultra high performance liquid chromatography-tandem mass spectrometry". Environmental Science: Processes & Impacts 20 (12): 1716–1727. December 2018. doi:10.1039/C8EM00390D. PMID 30350841.
- ↑ 154.0 154.1 "Practical Insights Into Improving Adherence to Metformin Therapy in Patients With Type 2 Diabetes". Clinical Diabetes 37 (3): 234–241. July 2019. doi:10.2337/cd18-0063. PMID 31371854.
- ↑ 155.0 155.1 "Effect of metformin on prostate cancer outcomes after radical prostatectomy". Urologic Oncology 32 (1): 43.e1–7. January 2014. doi:10.1016/j.urolonc.2013.05.005. PMID 23810664. "Metformin use at time of RP was extracted from the Mayo Clinic electronic medical record (EMR) by searching in the 3 months prior to the RP for the terms- metformin, Glucophage, Glumetza, Riomet, Fortamet, Obimet, Gluformin, Dianben, Diabex, Diaformin or Metsol.".
- ↑ 156.0 156.1 156.2 "Metformin". https://www.drugs.com/international/metformin.html.
- ↑ "Fixed-dose single tablet antidiabetic combinations". Diabetes, Obesity & Metabolism 11 (6): 527–33. June 2009. doi:10.1111/j.1463-1326.2008.00993.x. PMID 19175373.
- ↑ "Current therapeutic options in type 2 diabetes mellitus: a practical approach". Clinical Medicine & Research 1 (3): 189–200. July 2003. doi:10.3121/cmr.1.3.189. PMID 15931309.
- ↑ "FDA Approves GlaxoSmithKline's Avandamet (rosiglitazone maleate and metformin HCl), The Latest Advancement in the Treatment of Type 2 Diabetes" (Press release). GlaxoSmithKline. 12 October 2002. Archived from the original on 21 January 2007. Retrieved 27 December 2006.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=077337.
- ↑ "2009 Top 200 branded drugs by total prescriptions". http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard//drugtopics/252010/674969/article.pdf. (96.5 KB). Drug Topics (17 June 2010). Retrieved 2 September 2010.
- ↑ "Questions and Answers about the Seizure of Paxil CR and Avandamet" (Press release). U.S. Food and Drug Administration (FDA). 4 March 2005. Archived from the original on 14 October 2007. Retrieved 27 December 2006.
- ↑ "Teva Pharm announces settlement of generic Avandia, Avandamet, and Avandaryl litigation with GlaxoSmithKline" (Press release). Reuters. 27 September 2007. Archived from the original on 3 May 2021. Retrieved 17 February 2009.
- ↑ "Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes". The New England Journal of Medicine 356 (24): 2457–71. June 2007. doi:10.1056/NEJMoa072761. PMID 17517853.
- ↑ "European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim". European Medicines Agency (EMA). 23 September 2010. https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-suspension-avandia-avandamet-avaglim.
- ↑ "Call to 'suspend' diabetes drug". BBC News Online. 23 September 2010. https://www.bbc.co.uk/news/health-11397645.
- ↑ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. http://cdsco.nic.in/html/drugsbanned.html.
- ↑ "Diabetes drug withdrawn". Stuff.co.nz. NZPA. 17 February 2011. http://www.stuff.co.nz/4669573.
- ↑ "Controversial Diabetes Drug Harms Heart, U.S. Concludes". The New York Times. 19 February 2010. https://www.nytimes.com/2010/02/20/health/policy/20avandia.html.
- ↑ "Updated Risk Evaluation and Mitigation Strategy (REMS)". 1 July 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-updated-risk-evaluation-and-mitigation-strategy-rems-restrict-access.
- ↑ "FDA Drug Safety Communication: FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines". 21 June 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-removal-some-prescribing-and-dispensing-restrictions.
- ↑ "Glaxo's Avandia Cleared From Sales Restrictions by FDA". Bloomberg. https://www.bloomberg.com/news/2013-11-25/glaxo-s-avandia-cleared-from-sales-restrictions-by-fda.html.
- ↑ "FDA requires removal of certain restrictions on the diabetes drug Avandia". U.S. Food and Drug Administration (FDA) (Press release). 25 November 2013. Archived from the original on 4 May 2015.
- ↑ "US agency reverses stance on controversial diabetes drug". http://blogs.nature.com/news/2013/11/fda-reverses-stance-on-controversial-diabetes-drug.html.
- ↑ "Glubrava EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/glubrava.
- ↑ "Competact EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/competact.
- ↑ "Pioglitazone (marketed as Actos, Actoplus Met, Duetact, and Oseni) Information". 11 January 2017. http://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/pioglitazone-marketed-actos-actoplus-met-duetact-and-oseni-information.
- ↑ "FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function". 3 April 2013. http://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain.
- ↑ "FDA Drug Safety Communication: Updated FDA review concludes that use of type 2 diabetes medicine pioglitazone may be linked to an increased risk of bladder cancer". 4 August 2011. http://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-updated-fda-review-concludes-use-type-2-diabetes-medicine-pioglitazone.
- ↑ "Janumet- sitagliptin and metformin hydrochloride tablet, film coated". 12 August 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d19c7ed0-ad5c-426e-b2df-722508f97d67.
- ↑ "Janumet EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/janumet.
- ↑ "Kombiglyze XR- saxagliptin and metformin hydrochloride tablet, film coated, extended release". 24 October 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fbd25da4-ebe6-45c9-beb8-93523d11a0b4.
- ↑ "Komboglyze EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/komboglyze.
- ↑ "Kazano- alogliptin and metformin hydrochloride tablet, film coated". 14 June 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=83cb7914-a683-47bb-a713-f2bc6a596bd2.
- ↑ "Vipdomet EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/vipdomet.
- ↑ "Jentadueto EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/jentadueto.
- ↑ "Jentadueto- linagliptin and metformin hydrochloride tablet, film coated". 18 July 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6dd9b86-0d18-95d4-2bc7-05591bfdd597.
- ↑ "Jentadueto XR- linagliptin and metformin hydrochloride tablet, film coated, extended release". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d02a4d4-d312-80b4-05c4-691b8f0aa7aa.
- ↑ "Linagliptin and Metformin Hydrochloride: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208336.
- ↑ 190.0 190.1 "Progressing From Metformin to Sulfonylureas or Meglitinides" (in en-US). Workplace Health & Safety 64 (9): 433–9. September 2016. doi:10.1177/2165079916644263. PMID 27621259.
- ↑ 191.0 191.1 "Metformin and second- or third-generation sulphonylurea combination therapy for adults with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews 4 (4): CD012368. April 2019. doi:10.1002/14651858.CD012368.pub2. PMID 30998259.
- ↑ "The Use of Medicines in the United States: Review of 2010". http://www.imshealth.com/deployedfiles/imshealth/Global/Content/IMS%20Institute/Static%20File/IHII_UseOfMed_report.pdf. (1.79 MB). IMS Institute for Healthcare Informatics (April 2011). Retrieved 28 April 2011.
- ↑ "Drug Approval Package: PrandiMet (repaglinide/metformin HCI fixed-dose combination) NDA 22386". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022386_prandimet_toc.cfm.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=200624.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210874.
- ↑ "Qternmet XR (dapagliflozin, saxagliptin, and metformin hydrochloride) extended-release tablets, for oral use Initial U.S. Approval: 2019". https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=567183.
- ↑ "Beneficial effects of triple drug combination of pioglitazone with glibenclamide and metformin in type 2 diabetes mellitus patients on insulin therapy". The Journal of the Association of Physicians of India 51: 1061–4. November 2003. PMID 15260389.
- ↑ "Statement from Janet Woodcock, M.D., director of FDA's Center for Drug Evaluation and Research, on impurities found in diabetes drugs outside the U.S.". 5 December 2019. http://www.fda.gov/news-events/press-announcements/statement-janet-woodcock-md-director-fdas-center-drug-evaluation-and-research-impurities-found.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Recalls and safety alerts". 5 December 2019. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/71831a-eng.php.
- ↑ "Laboratory Tests - Metformin". 3 February 2020. http://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-metformin.
- ↑ "FDA Updates and Press Announcements on NDMA in Metformin". 4 February 2020. http://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-metformin.
- ↑ "APO-Metformin (2020-02-04)". Health Canada. 4 February 2020. https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/72281r-eng.php.
- ↑ "Ranbaxy Metformin Product Recall (2020-02-26)". Health Canada. 26 February 2020. https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/72455r-eng.php/.
- ↑ "Jamp-Metformin Product Recall (2020-03-10)". Health Canada. 10 March 2020. https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/72565r-eng.php/.
- ↑ "FDA Alerts Patients and Health Care Professionals to Nitrosamine Impurity Findings in Certain Metformin Extended-Release Products" (Press release). U.S. Food and Drug Administration (FDA). 28 May 2020. Archived from the original on 22 March 2021. Retrieved 2 June 2020.
- ↑ "Questions and Answers: NDMA impurities in metformin products". 28 May 2020. https://www.fda.gov/drugs/drug-safety-and-availability/questions-and-answers-ndma-impurities-metformin-products.
- ↑ "Amneal Pharmaceuticals LLC Issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended Release Tablets, USP, 500 mg and 750 mg, Due to Detection of N-Nitrosodimethylamine (NDMA) Impurity". 29 May 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/amneal-pharmaceuticals-llc-issues-voluntary-nationwide-recall-metformin-hydrochloride-extended.
- ↑ "Apotex Corp. Issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets 500mg Due to the Detection of N-nitrosodimethylamine (NDMA)". 27 May 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/apotex-corp-issues-voluntary-nationwide-recall-metformin-hydrochloride-extended-release-tablets.
- ↑ "Teva Pharmaceuticals USA, Inc. Initiates Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets USP 500 mg and 750 mg Due to Detection of N-Nitrosodimethylamine (NDMA)". 2 June 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/teva-pharmaceuticals-usa-inc-initiates-voluntary-nationwide-recall-metformin-hydrochloride-extended.
- ↑ "Marksans Pharma Limited Issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets, USP 500mg, Due to the Detection of N-Nitrosodimethylamine (NDMA)". 2 June 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/marksans-pharma-limited-issues-voluntary-nationwide-recall-metformin-hydrochloride-extended-release.
- ↑ "Re: Docket No. FDA-2020-P-0978". U.S. Food and Drug Administration (FDA). 28 May 2020. https://downloads.regulations.gov/FDA-2020-P-0978-0007/attachment_1.pdf.
- ↑ 212.0 212.1 212.2 "Laboratory Tests - Metformin". 5 June 2020. https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-metformin.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Lupin Pharmaceuticals, Inc. Issues Voluntarily Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets, 500mg and 1000mg Due to the Detection of N-Nitrosodimethylamine (NDMA) Impurity" (Press release). Lupin Pharmaceuticals Inc. Archived from the original on 9 June 2021. Retrieved 9 July 2020 – via PR Newswire.
- ↑ "Bayshore Pharmaceuticals, LLC Issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets USP, 500 mg and 750 mg Due to the Detection of N-Nitrosodimethylamine (NDMA) Impurity". 19 August 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/bayshore-pharmaceuticals-llc-issues-voluntary-nationwide-recall-metformin-hydrochloride-extended.
- ↑ "The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials". Pharmacological Research 159: 104799. September 2020. doi:10.1016/j.phrs.2020.104799. PMID 32278041.
- ↑ "Diabetes drugs for nonalcoholic fatty liver disease: a systematic review". Systematic Reviews 8 (1): 295. November 2019. doi:10.1186/s13643-019-1200-8. PMID 31783920.
- ↑ "Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis". World Journal of Gastroenterology 24 (30): 3361–3373. August 2018. doi:10.3748/wjg.v24.i30.3361. PMID 30122876.
- ↑ "Metformin treatment to prevent early puberty in girls with precocious pubarche". The Journal of Clinical Endocrinology and Metabolism 91 (8): 2888–91. August 2006. doi:10.1210/jc.2006-0336. PMID 16684823.
- ↑ "Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence". The Journal of Clinical Endocrinology and Metabolism 96 (8): E1262-7. August 2011. doi:10.1210/jc.2011-0555. PMID 21632811.
- ↑ "Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism". Reviews in Endocrine & Metabolic Disorders 10 (1): 63–76. March 2009. doi:10.1007/s11154-008-9096-y. PMID 18726694.
- ↑ "Metformin in cancer therapy: a new perspective for an old antidiabetic drug?". Molecular Cancer Therapeutics 9 (5): 1092–9. May 2010. doi:10.1158/1535-7163.MCT-09-1186. PMID 20442309.
- ↑ "Risk of cancer in diabetes: the effect of metformin". ISRN Endocrinology 2013: 636927. September 2013. doi:10.1155/2013/636927. PMID 24224094.
- ↑ 223.0 223.1 "Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis". Ageing Research Reviews 40: 31–44. November 2017. doi:10.1016/j.arr.2017.08.003. PMID 28802803.
- ↑ "Metformin as Anti-Aging Therapy: Is It for Everyone?". Trends in Endocrinology and Metabolism 30 (10): 745–755. October 2019. doi:10.1016/j.tem.2019.07.015. PMID 31405774.
- ↑ "Metformin for Treatment of Fragile X Syndrome and Other Neurological Disorders". Annual Review of Medicine 70: 167–181. January 2019. doi:10.1146/annurev-med-081117-041238. PMID 30365357. https://escholarship.mcgill.ca/concern/articles/2j62s977h. Retrieved 9 October 2022.
- ↑ "Metformin as a Tool to Target Aging". Cell Metabolism 23 (6): 1060–1065. June 2016. doi:10.1016/j.cmet.2016.05.011. PMID 27304507.
- ↑ Lee CG, Heckman-Stoddard B, Dabelea D, et al. Effect of Metformin and Lifestyle Interventions on Mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2021;44(12):2775-2782. doi:10.2337/dc21-1046
- ↑ "A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan". Frontiers in Endocrinology 12: 718942. 2021. doi:10.3389/fendo.2021.718942. PMID 34421827.
Further reading
- "Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics". Current Pharmaceutical Design 23 (17): 2532–2550. 2017. doi:10.2174/1381612822666161201152941. PMID 27908266.
- "Metformin and the gastrointestinal tract". Diabetologia 59 (3): 426–35. March 2016. doi:10.1007/s00125-015-3844-9. PMID 26780750.
- "Review of Metformin Use for Type 2 Diabetes Prevention". American Journal of Preventive Medicine 55 (4): 565–574. October 2018. doi:10.1016/j.amepre.2018.04.038. PMID 30126667.
- "The mechanisms of action of metformin". Diabetologia 60 (9): 1577–1585. September 2017. doi:10.1007/s00125-017-4342-z. PMID 28776086.
- "Metformin: clinical use in type 2 diabetes". Diabetologia 60 (9): 1586–1593. September 2017. doi:10.1007/s00125-017-4336-x. PMID 28770321.
- "Metformin: An Old Drug with New Applications". International Journal of Molecular Sciences 19 (10): 2863. September 2018. doi:10.3390/ijms19102863. PMID 30241400.
- "A preclinical overview of metformin for the treatment of type 2 diabetes". Biomedicine & Pharmacotherapy 106: 1227–1235. October 2018. doi:10.1016/j.biopha.2018.07.085. PMID 30119191.
External links
- "Nitrosamine impurities in medications: Guidance". 4 April 2022. https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities/medications-guidance.html.
 |