Chemistry:Porfimer sodium
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | ~90% |
| Elimination half-life | 21.5 days (mean) |
| Excretion | Fecal |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
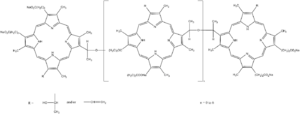
| Formula | C68H74N8O11 (for n=0) |
| Molar mass | 1179.36 g/mol (for n=0) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Porfimer sodium, sold as Photofrin, is a photosensitizer used in photodynamic therapy and radiation therapy and for palliative treatment of obstructing endobronchial non-small cell lung carcinoma and obstructing esophageal cancer.
Porfimer is a mixture of oligomers formed by ether and ester linkages of up to eight porphyrin units.[1] In practice, a red light source emitting at 630 nm is used to excite the Porfimer oligomers.[2]
Porfimer is Haematoporphyrin Derivative (HpD) (See PDT).
Approvals and indications
It was approved in Canada in 1993 for the treatment of bladder cancer.[2] It was approved in Japan in 1994 (for early stage lung cancer?).[2] It was approved by the U.S. FDA in December 1995 for esophageal cancer, and in 1998, it was approved for the treatment of early non-small cell lung cancer.[2]
In August 2003 the FDA approved its use for Barrett's esophagus.[3]
References
- ↑ "Porfimer injection Prescribing information". http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020451s019lbl.pdf.
- ↑ 2.0 2.1 2.2 2.3 "Photodynamic therapy (PDT) for lung cancers". Journal of Thoracic Oncology 1 (5): 489–93. June 2006. doi:10.1016/S1556-0864(15)31616-6. PMID 17409904.
- ↑ "FDA Patient Safety News: Show #20, October 2003". October 2003. http://www.accessdata.fda.gov/psn/printer-full.cfm?id=24.
External links
 |

