Biology:TGF beta receptor 2
 Generic protein structure example |
Transforming growth factor, beta receptor II (70/80kDa) is a TGF beta receptor. TGFBR2 is its human gene.
It is a tumor suppressor gene.[1]
Function
This gene encodes a member of the serine/threonine protein kinase family and the TGFB receptor subfamily. The encoded protein is a transmembrane protein that has a protein kinase domain, forms a heterodimeric complex with another receptor protein, and binds TGF-beta. This receptor/ligand complex phosphorylates proteins, which then enter the nucleus and regulate the transcription of a subset of genes related to cell proliferation. Mutations in this gene have been associated with Marfan syndrome, Loeys-Deitz aortic aneurysm syndrome, Osler–Weber–Rendu syndrome, and the development of various types of tumors. At least 73 disease-causing mutations in this gene have been discovered.[2] Alternatively spliced transcript variants encoding different isoforms have been characterized.[3]
Interactions
TGF beta receptor 2 has been shown to interact with:
- AP2B1,[4]
- Cyclin B2,[5]
- Endoglin,[6][7]
- Heat shock protein 90kDa alpha (cytosolic), member A1[8]
- STRAP,[9][10]
- TGF beta receptor 1,[11][12] and
- Transforming growth factor, beta 3.[6][13][14][15]
Domain architecture
| Transforming growth factor beta receptor 2 ectodomain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
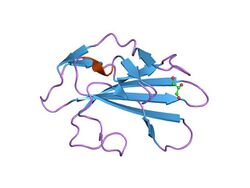 crystal structure of human tgf-beta type ii receptor ligand binding domain | |||||||||
| Identifiers | |||||||||
| Symbol | ecTbetaR2 | ||||||||
| Pfam | PF08917 | ||||||||
| InterPro | IPR015013 | ||||||||
| |||||||||
TGF beta receptor 2 consists of a C-terminal protein kinase domain and an N-terminal ectodomain. The ectodomain consists of a compact fold containing nine beta-strands and a single helix stabilised by a network of six intra strand disulphide bonds. The folding topology includes a central five-stranded antiparallel beta-sheet, eight-residues long at its centre, covered by a second layer consisting of two segments of two-stranded antiparallel beta-sheets (beta1-beta4, beta3-beta9).[14]
See also
- TGF beta receptors
References
- ↑ "TGFBR2 - transforming growth factor, beta receptor II (70/80kDa) - Genetics Home Reference". http://ghr.nlm.nih.gov/gene=tgfbr2.
- ↑ "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports 9 (1): 18577. December 2019. doi:10.1038/s41598-019-54976-4. PMID 31819097. Bibcode: 2019NatSR...918577S.
- ↑ "Entrez Gene: TGFBR2 transforming growth factor, beta receptor II (70/80kDa)". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=7048.
- ↑ "Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit". Molecular Biology of the Cell 13 (11): 4001–12. Nov 2002. doi:10.1091/mbc.02-07-0104. PMID 12429842.
- ↑ "Functional association of TGF-beta receptor II with cyclin B". Oncogene 18 (1): 269–75. Jan 1999. doi:10.1038/sj.onc.1202263. PMID 9926943.
- ↑ 6.0 6.1 "Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily". The Journal of Biological Chemistry 274 (2): 584–94. Jan 1999. doi:10.1074/jbc.274.2.584. PMID 9872992.
- ↑ "Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II". The Journal of Biological Chemistry 277 (32): 29197–209. Aug 2002. doi:10.1074/jbc.M111991200. PMID 12015308.
- ↑ "Critical regulation of TGFbeta signaling by Hsp90". Proceedings of the National Academy of Sciences of the United States of America 105 (27): 9244–9. Jul 2008. doi:10.1073/pnas.0800163105. PMID 18591668. Bibcode: 2008PNAS..105.9244W.
- ↑ "Identification of STRAP, a novel WD domain protein in transforming growth factor-beta signaling". The Journal of Biological Chemistry 273 (52): 34671–4. Dec 1998. doi:10.1074/jbc.273.52.34671. PMID 9856985.
- ↑ "STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling". Molecular and Cellular Biology 20 (9): 3157–67. May 2000. doi:10.1128/mcb.20.9.3157-3167.2000. PMID 10757800.
- ↑ "Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor". The Journal of Biological Chemistry 270 (10): 5625–30. Mar 1995. doi:10.1074/jbc.270.10.5625. PMID 7890683.
- ↑ "Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor". The Journal of Biological Chemistry 276 (9): 6727–38. Mar 2001. doi:10.1074/jbc.M008340200. PMID 11102446.
- ↑ "Transforming growth factor-beta (TGF-beta) binding to the extracellular domain of the type II TGF-beta receptor: receptor capture on a biosensor surface using a new coiled-coil capture system demonstrates that avidity contributes significantly to high affinity binding". Journal of Molecular Biology 328 (5): 1173–83. May 2003. doi:10.1016/s0022-2836(03)00360-7. PMID 12729750.
- ↑ 14.0 14.1 "Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex". Nature Structural Biology 9 (3): 203–8. Mar 2002. doi:10.1038/nsb766. PMID 11850637.
- ↑ "Type III TGF-beta receptor-independent signalling of TGF-beta2 via TbetaRII-B, an alternatively spliced TGF-beta type II receptor". The EMBO Journal 20 (3): 480–90. Feb 2001. doi:10.1093/emboj/20.3.480. PMID 11157754.
External links
 |