Chemistry:Crotonyl-CoA
| |
| Names | |
|---|---|
| IUPAC name
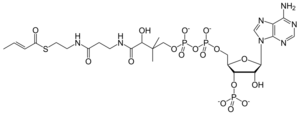
3′-O-Phosphonoadenosine 5′-[(3R)-4-({3-[(2-{[(2E)-but-2-enoyl]sulfanyl}ethyl)amino]-3-oxopropyl}amino)-3-hydroxy-2,2-dimethyl-4-oxobutyl dihydrogen diphosphate]
| |
| Systematic IUPAC name
[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl (3R)-4-({3-[(2-{[(2E)-but-2-enoyl]sulfanyl}ethyl)amino]-3-oxopropyl}amino)-3-hydroxy-2,2-dimethyl-4-oxobutyl dihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | Crotonyl-coenzyme+A |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C25H40N7O17P3S | |
| Molar mass | 835.609 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Crotonyl-coenzyme A is an intermediate in the fermentation of butyric acid, and in the metabolism of lysine and tryptophan.[1] It is important in the metabolism of fatty acids and amino acids.[2]
Crotonyl-coA and reductases
Before a 2007 report by Alber and coworkers, crotonyl-coA carboxylases and reductases (CCRs) were known for reducing crotonyl-coA to butyryl-coA.[3] A report by Alber and coworkers concluded that a specific CCR homolog was able to reduce crotonyl-coA to (2S)-ethyl malonyl-coA which was a favorable reaction.[3] The specific CCR homolog came from the bacterium Rhodobacter sphaeroides.[3]
Role of Crotonyl-coA in Transcription
Post-translational modification of histones either by acetylation or crotonylation is important for the active transcription of genes.[4] Histone crotonylation is regulated by the concentration of crotonyl-coA which can change based on environmental cell conditions or genetic factors.[4]
References
- ↑ Ray, Lauren; Valentic, Timothy R; Miyazawa, Takeshi; Withall, David M; Song, Lijiang; Milligan, Jacob C; Osada, Hiroyuki; Takahashi, Shunji et al. (2016). "A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units". Nature Communications 7: 13609. doi:10.1038/ncomms13609. PMID 28000660. Bibcode: 2016NatCo...713609R.
- ↑ "Crotonyl-CoA". https://pubchem.ncbi.nlm.nih.gov/compound/5280381#section=Top.
- ↑ 3.0 3.1 3.2 Wilson, Micheal C.; Moore, Bradley S. (2012). "Beyond ethylmalonyl-CoA: The functional role of crotonyl-CoAcarboxylase/reductase homologs in expanding polyketide diversity". Nat. Prod. Rep. 29 (1): 72–86. doi:10.1039/c1np00082a. ISSN 0265-0568. PMID 22124767. http://dx.doi.org/10.1039/c1np00082a.
- ↑ 4.0 4.1 Sabari, Benjamin R.; Tang, Zhanyun; Huang, He; Yong-Gonzalez, Vladimir; Molina, Henrik; Kong, Ha Eun; Dai, Lunzhi; Shimada, Miho et al. (2015-04-16). "Intracellular Crotonyl-CoA Stimulates Transcription through p300-Catalyzed Histone Crotonylation" (in English). Molecular Cell 58 (2): 203–215. doi:10.1016/j.molcel.2015.02.029. ISSN 1097-2765. PMID 25818647.
See also
 |

