Chemistry:Urocanic acid
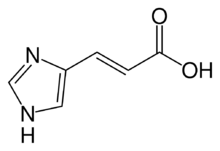
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-(1H-imidazol-4-yl)prop-2-enoic acid | |
| Other names
(E)-3-(1H-imidazol-4-yl)acrylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Urocanic+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H6N2O2 | |
| Molar mass | 138.124 g/mol |
| Melting point | 225 °C (437 °F; 498 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Urocanic acid is an intermediate in the catabolism of L-histidine.
Metabolism
It is formed from L-histidine through the action of histidine ammonialyase (also known as histidase or histidinase) by elimination of ammonium.
In the liver, urocanic acid is transformed by urocanate hydratase (or urocanase) to 4-imidazolone-5-propionic acid and subsequently to glutamic acid.
Clinical significance
Inherited deficiency of urocanase leads to elevated levels of urocanic acid in the urine, a condition known as urocanic aciduria.
An important role for the onset of atopic dermatitis and asthma has been attributed to filaggrin, a skin precursor of urocanic acid.[1][2]
Urocanic acid is thought to be a significant attractant of the nematode parasite Strongyloides stercoralis,[3] in part because of relatively high levels in the plantar surfaces of the feet, the site through which this parasite often enters the body.
Function
Urocanic acid was detected in animal sweat and skin where, among other possible functions, it acts as an endogenous sunscreen or photoprotectant against UVB-induced DNA damage. Urocanic acid is found predominantly in the stratum corneum of the skin and it is likely that most of it is derived from filaggrin catabolism (a histidine-rich protein). When exposed to UVB irradiation, trans-urocanic acid is converted in vitro and in vivo to the cis isomer.[4] The cis form is known to activate regulatory T cells.[5]
Some studies attribute filaggrin an important role in keeping the skin surface slightly acidic, through a breaking down mechanism to form histidine and subsequently trans-urocanic acid,[6] however others have shown that the filaggrin–histidine–urocanic acid cascade is not essential for skin acidification.[7]
History
Urocanic acid was first isolated in 1874 by the chemist Max Jaffé from the urine of a dog,[8][9] hence the name (Latin: urina = urine, and canis = dog).
See also
- Histidinemia
- Inborn error of metabolism
References
- ↑ "The Pathogenetic Effect of Natural and Bacterial Toxins on Atopic Dermatitis". Toxins 9 (1): 3. December 2016. doi:10.3390/toxins9010003. PMID 28025545.
- ↑ "Filaggrin mutations associated with skin and allergic diseases". The New England Journal of Medicine 365 (14): 1315–27. October 2011. doi:10.1056/nejmra1011040. PMID 21991953.
- ↑ Safer, D.; Brenes, M.; Dunipace, S.; Schad, G. (2007). "Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis". Proceedings of the National Academy of Sciences 104 (5): 1627–1630. doi:10.1073/pnas.0610193104. PMID 17234810.
- ↑ "The evaluation of the amount of cis- and trans-urocanic acid in the stratum corneum by Raman spectroscopy". Photochemical and Photobiological Sciences 9 (5): 730–3. May 2010. doi:10.1039/b9pp00143c. PMID 20442934.
- ↑ "Mechanisms of UV-induced immunosuppression". The Keio Journal of Medicine 54 (4): 165–71. December 2005. doi:10.2302/kjm.54.165. PMID 16452825. http://www.kjm.keio.ac.jp/past/54/4/165.pdf.
- ↑ "Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema". Allergy 65 (7): 911–8. July 2010. doi:10.1111/j.1398-9995.2010.02326.x. PMID 20132155.
- ↑ "Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification?". The Journal of Investigative Dermatology 130 (8): 2141–4. August 2010. doi:10.1038/jid.2010.74. PMID 20376063.
- ↑ "Ueber die Urocaninsäure" (in German). Berichte der Deutschen Chemischen Gesellschaft 8 (1): 811–813. 1875. doi:10.1002/cber.187500801267. https://zenodo.org/record/1425082.
- ↑ "Ueber einen neuen Bestandtheil des Hundeharns" (in German). Berichte der Deutschen Chemischen Gesellschaft 7 (2): 1669–1673. 1874. doi:10.1002/cber.187400702225. https://zenodo.org/record/1425064.
External links
- The Online Metabolic and Molecular Bases of Inherited Disease - Chapter 80 - An overview of disorders of histidine metabolism, including urocanic aciduria.
 |

