Chemistry:Eganelisib
| |||
| Clinical data | |||
|---|---|---|---|
| Pregnancy category |
| ||
| Routes of administration | Oral | ||
| ATC code |
| ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEMBL | |||
| PDB ligand | |||
| Chemical and physical data | |||
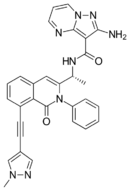
| Formula | C30H24N8O2 | ||
| Molar mass | 528.576 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
Eganelisib (USAN), codenamed IPI-549, is an experimental drug being investigated as a possible treatment for cancer. It is a highly selective phosphoinositide 3-kinase inhibitor, and thus works by inhibiting the enzyme PIK3CG, disrupting the PI3K/AKT/mTOR signaling pathway which plays important roles in the development of cancer.[1]
Eganelisib is being developed by Infinity Pharmaceuticals. Early clinical trial results were published in September 2016.[2] On September 29, 2020, it was granted Fast Track designation by the United States Food and Drug Administration (FDA) as a treatment for inoperable, locally advanced, or metastatic triple-negative breast cancer, combined with a checkpoint inhibitor and chemotherapy.[3]
(As of October 2020), five phase I/II clinical trials were ongoing in the United States, and one in Europe.[4]
References
- ↑ "Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate". ACS Med Chem Lett 7 (9): 862–7. September 2016. doi:10.1021/acsmedchemlett.6b00238. PMID 27660692.
- ↑ Corey Williams (2016-09-27). "Infinity Pharmaceuticals Inc. Presents Initial Clinical And New Preclinical Data On IPI-549 At Second CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference". The Smarter Analyst. https://www.smarteranalyst.com/stock-news/company-update-nasdaqinfi-infinity-pharmaceuticals-inc-presents-initial-clinical-new-preclinical-data-ipi-549-second-cri-cimt-eati-aacr-international-cancer-immunotherapy-conference/.
- ↑ "Infinity Receives Fast Track Designation for Eganelisib in Combination with a Checkpoint Inhibitor and Chemotherapy for First". Bloomberg (Press release). 2020-09-29. Retrieved 2020-10-30.
- ↑ "CID 91933883 | C30H24N8O2 - PubChem: ClinicalTrials.gov". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/91933883#section=ClinicalTrials-gov&fullscreen=true.
Further reading
- "Discovery of Potent and Selective PI3Kγ Inhibitors". J Med Chem 63 (19): 11235–11257. October 2020. doi:10.1021/acs.jmedchem.0c01203. PMID 32865410.
 |