Chemistry:Indium(III) iodide
| |
| Names | |
|---|---|
| Other names
Indium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| InI3 | |
| Molar mass | 495.53 g/mol |
| Appearance | Yellow solid |
| Density | 4.69 g/cm3 |
| Melting point | 210 °C (410 °F; 483 K) |
| Boiling point | 500 °C (932 °F; 773 K) |
| Related compounds | |
Other anions
|
Indium(III) bromide Indium(III) chloride |
Other cations
|
Aluminum iodide Gallium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.
Preparation
Indium(III) iodide can be obtained by reacting indium with iodine vapor:[1]
- 2 In + 3I
2 → 2 InI
3
Indium(III) iodide can also be obtained by evaporation of a solution of indium in HI.[2]
Properties
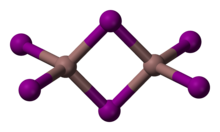
Indium(III) iodide is a pale yellow, very hygroscopic monoclinic solid (space group P21/c (space group no. 14), a = 9.837 Å, b = 6.102 Å, c = 12.195 Å, β = 107.69°),[3] which melts at 210 °C to form a dark brown liquid and is highly soluble in water. Its crystals consist of dimeric molecules.[4] The yellow β form slowly converts to the red α form.[5] In the presence of water vapor, the compound reacts with oxygen at 245 °C to form indium(III) oxide iodide.[6]
Distinct yellow and red forms are known. The red form undergoes a transition to the yellow at 57 °C. The structure of the red form has not been determined by X-ray crystallography; however, spectroscopic evidence indicates that indium may be six coordinate.[7] The yellow form consists of In2I6 with 4 coordinate indium centres.
References
- ↑ Handbuch der präparativen anorganischen Chemie. 1 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1975. ISBN 978-3-432-02328-1.
- ↑ E. Donges (1963). "Indium(III) Iodide". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 861–2.
- ↑ Forrester, J. D.; Zalkin, Allan; Templeton, David H. (Jan 1964). "Crystal and Molecular Structure of Indium(III) Iodide (In 2 I 6 )" (in en). Inorganic Chemistry 3 (1): 63–67. doi:10.1021/ic50011a013. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic50011a013.
- ↑ Handbuch der präparativen anorganischen Chemie. 1 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1975. ISBN 978-3-432-02328-1.
- ↑ Downs, Anthony John (1993). Chemistry of aluminium, gallium, indium and thallium. London Glasgow New York [etc.]: Blackie. ISBN 978-0-7514-0103-5.
- ↑ Hagen, A. P. (2009-09-17) (in en). Inorganic Reactions and Methods, The Formation of Bonds to Group VIB (O, S, Se, Te, Po) Elements (Part 1). John Wiley & Sons. ISBN 978-0-470-14540-1. https://books.google.com/books?id=pDfY5xkvxeYC&pg=PA301.
- ↑ Taylor M. J., Kloo L. A. Journal of Raman Spectroscopy 31, 6, (2000), 465
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

