Chemistry:Phytomenadione
 | |
| Clinical data | |
|---|---|
| Trade names | Mephyton, others |
| Other names | Vitamin K1, phytonadione, phylloquinone, (E)-phytonadione |
| AHFS/Drugs.com | Monograph |
| Routes of administration | By mouth, subcutaneous, intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
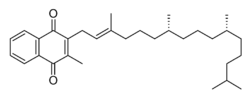
| Formula | C31H46O2 |
| Molar mass | 450.707 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phytomenadione, also known as vitamin K1 or phylloquinone, is a vitamin found in food and used as a dietary supplement.[2][3] It is on the World Health Organization's List of Essential Medicines.[4]
As a supplement it is used to treat certain bleeding disorders.[3] This includes warfarin overdose, vitamin K deficiency, and obstructive jaundice.[3] It is also recommended to prevent and treat vitamin K deficiency bleeding in infants.[3] Use is typically recommended by mouth, intramuscular injection or injection under the skin.[3] When given by injection benefits are seen within two hours.[3] Many countries in the world choose intramuscular injections in newborn to keep them safe from severe bleeding (VKDB). It is considered a safe treatment and saves many children from death and severe neurologic deficit every year.[5]
Side effects when given by injection may include pain at the site of injection.[3] Severe allergic reactions may occur when it is injected into a vein or muscle, but this has mainly happened when large doses of a certain type of supplement containing castor oil were given intravenously.[6] Use during pregnancy is considered safe,[7] use is also likely okay during breastfeeding.[8] It works by supplying a required component for making a number of blood clotting factors.[3] Food sources include green vegetables, vegetable oil, and some fruit.[9]
Phytomenadione was first isolated in 1939.[10] In 1943 Edward Doisy and Henrik Dam were given a Nobel Prize for its discovery.[10]
Terminology
Phytomenadione is often also called phylloquinone, vitamin K,[11] or phytonadione.
A stereoisomer of phylloquinone is called vitamin k1 (note the difference in capitalization).[citation needed]
Chemistry
Vitamin K is a fat-soluble vitamin that is stable in air and moisture but decomposes in sunlight.[12] It is a polycyclic aromatic ketone, based on 2-methyl-1,4-naphthoquinone, with a 3-phytyl substituent. It is found naturally in a wide variety of green plants, particularly in leaves, since it functions as an electron acceptor during photosynthesis, forming part of the electron transport chain of photosystem I.[13][14]
Biological function
Animals
The best-known function of vitamin K in animals is as a cofactor in the formation of coagulation factors II (prothrombin), VII, IX, and X by the liver. It is also required for the formation of anticoagulant factors protein C and S. It is commonly used to treat warfarin toxicity, and as an antidote for coumatetralyl.
Vitamin K is required for bone protein formation.
In terms of distribution, phylloquinone typically occurs in higher levels in the liver, heart and pancreas, but in lower levels in the brain, kidneys, and lungs.[15]
Plants and cyanobacteria
Vitamin K1 is required for plant photosynthesis, where it participates in the Photosystem I electron transport chain.[16]
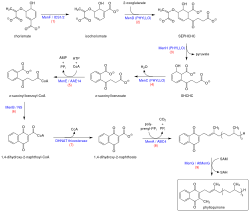
Biosynthesis
Vitamin K1 is synthesized from chorismate, a compound produced from shikimate via the shikimate pathway. The conversion of chorismate to vitamin K1 comprises a series of nine steps:[17][18][19]
- Chorismate is isomerized to isochorismate by isochorismate synthase, or MenF (menaquinone enzyme).
- Addition of 2-oxoglutarate to isochorismate by PHYLLO, a multifunctional protein comprising three different enzymatic activities (MenD, H, and C).
- Elimination of pyruvate by PHYLLO.
- Aromatization to yield o-succinyl benzoate by PHYLLO.
- O-succinylbenzoate activation to corresponding CoA ester by MenE.
- Naphthoate ring formation by naphthoate synthase (MenB/NS).
- Thiolytic release of CoA by a thioesterase (MenH).
- Attachment of phytol chain to the naphthoate ring (MenA/ABC4).
- Methylation of the precursor at position 3 (MenG).
See also
References
- ↑ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-08-30"]. 26 October 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/multiple-additions-2023-08-30.html.
- ↑ (in en) Diet and Exercise in Cystic Fibrosis. Academic Press. 2014. p. 187. ISBN 9780128005880. https://books.google.com/books?id=hSSOAwAAQBAJ&pg=PA187.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Phytonadione". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/phytonadione.html.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "American Academy of Pediatrics on vitamin k in the newborn period". 27 July 2018. https://www.aappublications.org/news/2018/07/27/vitamin-k-in-the-newborn-period-how-important-is-it-pediatrics-7-27-18.
- ↑ "The incidence of anaphylaxis following intravenous phytonadione (vitamin K1): a 5-year retrospective review". Annals of Allergy, Asthma & Immunology 89 (4): 400–406. October 2002. doi:10.1016/S1081-1206(10)62042-X. PMID 12392385.
- ↑ "Are there teratogenic risks associated with antidotes used in the acute management of poisoned pregnant women?". Birth Defects Research. Part A, Clinical and Molecular Teratology 67 (2): 133–140. February 2003. doi:10.1002/bdra.10007. PMID 12769509.
- ↑ "Phytonadione Use During Pregnancy". https://www.drugs.com/pregnancy/phytonadione.html.
- ↑ "Vitamin K". Office of Dietary Supplements. U.S. National Institutes of Health. 11 February 2016. https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional.
- ↑ 10.0 10.1 Sneader, Walter (2005) (in en). Drug Discovery: A History. John Wiley & Sons. p. 243. ISBN 9780471899792. https://books.google.com/books?id=Cb6BOkj9fK4C&pg=PA243.
- ↑ "The content of phylloquinone (vitamin K1) in human milk, cows' milk and infant formula foods determined by high-performance liquid chromatography". The Journal of Nutrition 112 (6): 1105–1117. June 1982. doi:10.1093/jn/112.6.1105. PMID 7086539.
- ↑ Institute of Medicine (US) Panel on Micronutrients. "Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc". Washington (DC): National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK222299/#ddd00194.
- ↑ "Vitamin K1 (Phylloquinone) Restores the Turnover of FeS centers of Ether-extracted Spinach PSI Particles". FEBS Letters 243 (1): 47–52. 1989. doi:10.1016/0014-5793(89)81215-3.
- ↑ "Is phylloquinone an obligate electron carrier in photosystem I?". FEBS Letters 215 (1): 58–62. May 1987. doi:10.1016/0014-5793(87)80113-8. PMID 3552735.
- ↑ "Relationship between Structure and Biological Activity of Various Vitamin K Forms". Foods 10 (12): 3136. December 2021. doi:10.3390/foods10123136. PMID 34945687.
- ↑ "Phylloquinone (Vitamin K1): Occurrence, Biosynthesis and Functions". Mini Reviews in Medicinal Chemistry 17 (12): 1028–1038. 2017. doi:10.2174/1389557516666160623082714. PMID 27337968.
- ↑ "Biosynthesis of vitamin K1 (phylloquinone) by plant peroxisomes and its integration into signaling molecule synthesis pathways". Peroxisomes and their Key Role in Cellular Signaling and Metabolism. Subcellular Biochemistry. 69. Springer Netherlands. 2013. pp. 213–29. doi:10.1007/978-94-007-6889-5_12. ISBN 9789400768888.
- ↑ "A dedicated thioesterase of the Hotdog-fold family is required for the biosynthesis of the naphthoquinone ring of vitamin K1". Proceedings of the National Academy of Sciences of the United States of America 106 (14): 5599–5603. April 2009. doi:10.1073/pnas.0900738106. PMID 19321747. Bibcode: 2009PNAS..106.5599W.
- ↑ "Vitamin K1 (Phylloquinone)". Advances in Botanical Research 59: 229–261. 2011. doi:10.1016/B978-0-12-385853-5.00001-5. ISBN 9780123858535. https://www.researchgate.net/publication/285136704.
External links
 |