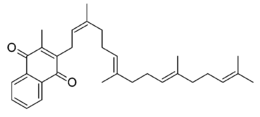
Chemistry:Menatetrenone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3-methyl-2-[(2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl]naphthalene-1,4-dione |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Low (oral)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C31H40O2 |
| Molar mass | 444.659 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Menatetrenone (INN), also known as menaquinone-4 (MK-4), is one of the nine forms of vitamin K2.
Biology
MK-4 is the major form of Vitamin K in vertebrate animals, including humans and common forms of meat animals. It is produced via conversion of vitamin K1 in the body, specifically in the testes, pancreas and arterial walls.[2] The conversion is not dependent on gut bacteria, occurring in germ-free rats[3][4] and in parenterally-administered K1 in rats.[5][6] Tissues that accumulate high amounts of MK-4 have a capacity to convert up to 90% of the available K1 into MK-4.[3][4][dubious ]
K1 is converted to MK-4 in three steps:[7]
- Removal of the phytyl tail to form menadione (K3; unknown enzyme);
- Reduction of menadione to menadiol (likely NQO1);
- Attachment of GGPP tail to form menaquinol-4, the reduced form of MK-4 (UBIAD1)
The second and third steps are known to happen in target tissue. The first step is proposed to happen mainly in the intestines.[7]
As a medication
Menatetrenone is approved in Japan for second-line treatment of postmenopausal osteoporosis. Evidence is restricted to small-scale RCTs; the minimum effective dose (for bone mass parameters) is 45 mg, much higher than the Daily Value for vitamin K (80 μg).[8]
Bioavailbility and dose
420 μg of oral MK-4, in a single-dose or spread out over 7 days, does not cause detectable changes in serum MK-4 level in healthy women, whereas MK-7 produces the expected increases in MK-7 levels.[1]
The minimum effective oral dose to change serum osteocalcin levels is 1500 μg/d, where as oral MK-7 is effective on this parameter at 45 μg/d, a level more in line with nutritional intake. In addition, rat studies show that oral MK-7 is better at increasing extrahepatic tissue levels of MK-4 than oral MK-4.[1]
References
- ↑ 1.0 1.1 1.2 "Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women". Nutrition Journal 11 (93): 93. November 2012. doi:10.1186/1475-2891-11-93. PMID 23140417.
- ↑ "Metabolism and cell biology of vitamin K". Thrombosis and Haemostasis 100 (4): 530–47. October 2008. doi:10.1160/TH08-03-0147. PMID 18841274.
- ↑ 3.0 3.1 "Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria". The Journal of Nutrition 128 (2): 220–3. February 1998. doi:10.1093/jn/128.2.220. PMID 9446847.
- ↑ 4.0 4.1 "Intestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the rat". Biochimica et Biophysica Acta (BBA) - General Subjects 1379 (1): 69–75. January 1998. doi:10.1016/S0304-4165(97)00089-5. PMID 9468334.
- ↑ "Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4". The British Journal of Nutrition 72 (3): 415–25. September 1994. doi:10.1079/BJN19940043. PMID 7947656.
- ↑ "Comparative metabolism and requirement of vitamin K in chicks and rats". The Journal of Nutrition 122 (12): 2354–60. December 1992. doi:10.1093/jn/122.12.2354. PMID 1453219.
- ↑ 7.0 7.1 "Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis". Journal of Lipid Research 55 (3): 345–362. March 2014. doi:10.1194/jlr.R045559. PMID 24489112.
- ↑ "Vitamin K2 therapy for postmenopausal osteoporosis". Nutrients 6 (5): 1971–80. May 2014. doi:10.3390/nu6051971. PMID 24841104. "administered daily doses of 15, 45, 90, and 135 mg revealed that 45 mg was the minimum effective dose for improving bone mass parameters evaluated by microdensitometry and/or single photon absorptiometry in postmenopausal women with osteoporosis".
 |
