Chemistry:Potassium sulfite
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Potassium sulfite
| |
| Other names
E225
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
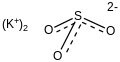
| K2SO3 | |
| Molar mass | 158.26 g/mol |
| Appearance | White solid |
| Density | 2.49 g/cm3[1] |
| Soluble | |
| Acidity (pKa) | 8 |
| −64.0·10−6 cm3/mol | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Potassium sulfate Potassium selenite |
Other cations
|
Sodium sulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Potassium sulfite is the inorganic compound with the formula K2SO3. It is the salt of potassium cation and sulfite anion. It is a white solid that is highly soluble in water. Potassium sulfite is widely used for preserving food and beverages.
Production and reactions
Potassium sulfite is produced by the thermal decomposition of potassium metabisulfite at 190°C:[2]
- K2S2O5 → K2SO3 + SO2
Structure
The structure of solid K
2SO
3, as assessed by X-ray crystallography. The S-O distances are 1.515 Å, and the O-S-O angles are 105.2°[1]
References
- ↑ 1.0 1.1 Andersen, Leif; Strömberg, Dan; Nevala, H.; Pohjola, S.; Niinistö, Lauri; Volden, Hans V.; Weidlein, Johann; Zingaro, Ralph A. (1986). "The Structure of Potassium Sulfite". Acta Chemica Scandinavica 40a: 479–480. doi:10.3891/acta.chem.scand.40a-0479.
- ↑ Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. 2. pp. 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
 |

