Biology:Cholecystokinin
Cholecystokinin (CCK or CCK-PZ; from Greek chole, "bile"; cysto, "sac"; kinin, "move"; hence, move the bile-sac (gallbladder)) is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of digestive enzymes and bile from the pancreas and gallbladder, respectively, and also acts as a hunger suppressant.[1][2]
History
Evidence that the small intestine controls the release of bile was uncovered as early as 1856, when French physiologist Claude Bernard showed that when dilute acetic acid was applied to the orifice of the bile duct, the duct released bile into the duodenum.[3][4] In 1903 the French physiologist Émile Wertheimer (fr) showed that this reflex was not mediated by the nervous system.[5] In 1904 the French physiologist Charles Fleig showed that the discharge of bile was mediated by a substance that was conveyed by the blood.[6] There remained the possibility that the increased flow of bile in response to the presence of acid in the duodenum might be due to secretin, which had been discovered in 1902. The problem was finally resolved in 1928 by Andrew Conway Ivy and his colleague Eric Oldberg of the Northwestern University Medical School, who found a new hormone that caused contraction of the gall bladder and that they called "cholecystokinin".[7] In 1943, Alan A. Harper and Henry S. Raper of the University of Manchester discovered a hormone that stimulated pancreatic enzyme secretion and that they named "pancreozymin";[8] however, pancreozymin was subsequently found to be cholecystokinin.[9][10][11] Swedish biochemists Johannes Erik Jorpes and Viktor Mutt undertook the monumental task of isolating and purifying porcine cholecystokinin and then determining its amino acid sequence. They finally presented porcine cholecystokinin's amino acid sequence in 1968.[12]
Structure
Cholecystokinin is a member of the gastrin/cholecystokinin family of peptide hormones and is very similar in structure to gastrin, another gastrointestinal hormone. CCK and gastrin share the same five C-terminal amino acids. CCK is composed of varying numbers of amino acids depending on post-translational modification of the 150-amino acid precursor, preprocholecystokinin.[13] Thus, the CCK peptide hormone exists in several forms, each identified by the number of amino acids it contains, e.g., CCK-58, CCK-33, CCK-22 and CCK-8. CCK58 assumes a helix-turn-helix configuration.[14] Biological activity resides in the C-terminus of the peptide. Most CCK peptides have a sulfate group attached to a tyrosine located seven residues from the C-terminus (see tyrosine sulfation).[13] This modification is crucial for the ability of CCK to activate the cholecystokinin A receptor. Nonsulfated CCK peptides also occur, which consequently cannot activate the CCK-A receptor, but their biological role remains unclear.[15][13]
Function
CCK plays important physiological roles both as a neuropeptide in the central nervous system and as a peptide hormone in the gut.[16] It is the most abundant neuropeptide in the central nervous system.[17][18] CCK has been researched thoroughly for its role in digestion[19] and it participates in a number of processes such as digestion, satiety and anxiety.[citation needed]
Gastrointestinal
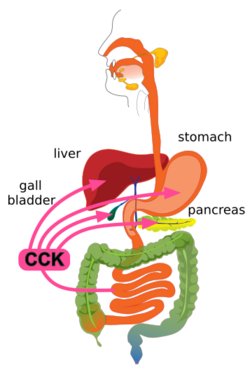
CCK is synthesized and released by enteroendocrine cells in the mucosal lining of the small intestine (mostly in the duodenum and jejunum), called I cells, neurons of the enteric nervous system, and neurons in the brain.[1] It is released rapidly into the circulation in response to a meal. The greatest stimulator of CCK release is the presence of fatty acids and/or certain amino acids in the chyme entering the duodenum.[13] In addition, release of CCK is stimulated by monitor peptide (released by pancreatic acinar cells), CCK-releasing protein (via paracrine signalling mediated by enterocytes in the gastric and intestinal mucosa), and acetylcholine (released by the parasympathetic nerve fibers of the vagus nerve).[20]
Once in the circulatory system, CCK has a relatively short half-life.[21]
Digestion
CCK mediates digestion in the small intestine by inhibiting gastric emptying. It stimulates the acinar cells of the pancreas to release a juice rich in pancreatic digestive enzymes (hence an alternate name, pancreozymin) that catalyze the digestion of fat, protein, and carbohydrates. Thus, as the levels of the substances that stimulated the release of CCK drop, the concentration of the hormone drops as well. The release of CCK is also inhibited by somatostatin and pancreatic peptide. Trypsin, a protease released by pancreatic acinar cells, hydrolyzes CCK-releasing peptide and monitor peptide, in effect turning off the additional signals to secrete CCK.[22]
CCK also causes the increased production of hepatic bile, and stimulates the contraction of the gall bladder and the relaxation of the sphincter of Oddi (Glisson's sphincter), resulting in the delivery of bile into the duodenal part of the small intestine.[1][2] Bile salts form amphipathic lipids, micelles that emulsify fats, aiding in their digestion and absorption.[1]
Satiety
As a peptide hormone, CCK mediates satiety by acting on the CCK receptors distributed widely throughout the central nervous system. The mechanism for hunger suppression is thought to be a decrease in the rate of gastric emptying.[23] CCK also has stimulatory effects on the vagus nerve, effects that can be inhibited by capsaicin.[24] The stimulatory effects of CCK oppose those of ghrelin, which has been shown to inhibit the vagus nerve.[25]
The effects of CCK vary between individuals. For example, in rats, CCK administration significantly reduces hunger in adult males, but is slightly less effective in younger subjects, and even slightly less effective in females. The hunger-suppressive effects of CCK also are reduced in obese rats.[26]
Neurological
CCK is found extensively throughout the central nervous system, with high concentrations found in the limbic system.[27] CCK is synthesized as a 115 amino acid preprohormone, that is then converted into multiple isoforms.[27] The predominant form of CCK in the central nervous system is the sulfated octapeptide, CCK-8S.[27]
Anxiogenic
In both humans and rodents, studies clearly indicate that elevated CCK levels causes increased anxiety.[21] The site of the anxiety-inducing effects of CCK seems to be central with specific targets being the basolateral amygdala, hippocampus, hypothalamus, periaqueductal grey, and cortical regions.[21][28]
Panicogenic
The CCK tetrapeptide fragment CCK-4 (Trp-Met-Asp-Phe-NH2) reliably causes anxiety and panic attacks (panicogenic effect) when administered to humans and is commonly used in scientific research for this purpose of in order to test new anxiolytic drugs.[28][29] Positron emission tomography visualization of regional cerebral blood flow in patients undergoing CCK-4 induced panic attacks show changes in the anterior cingulate gyrus, the claustrum-insular-amygdala region, and cerebellar vermis.[27]
Hallucinogenic
Several studies have implicated CCK as a cause of visual hallucinations in Parkinson's disease. Mutations in CCK receptors in combination with mutated CCK genes potentiate this association. These studies also uncovered potential racial/ethnic differences in the distribution of mutated CCK genes.[16]
Interactions
CCK has been shown to interact with the Cholecystokinin A receptor located mainly on pancreatic acinar cells and Cholecystokinin B receptor mostly in the brain and stomach. CCKB receptor also binds gastrin, a gastrointestinal hormone involved in stimulating gastric acid release and growth of the gastric mucosa.[30][31][32] CCK has also been shown to interact with calcineurin in the pancreas. Calcineurin will go on to activate the transcription factors NFAT 1–3, which will stimulate hypertrophy and growth of the pancreas. CCK can be stimulated by a diet high in protein, or by protease inhibitors.[33] CCK has been shown to interact with orexin neurons, which control appetite and wakefulness (sleep).[34] CCK can have indirect effects on sleep regulation.[35]
CCK in the body cannot cross the blood–brain barrier, but certain parts of the hypothalamus and brainstem are not protected by the barrier.
See also
- Antianalgesia
- Cholecystokinin antagonist
- Proglumide
References
- ↑ 1.0 1.1 1.2 1.3 Gastrointestinal Physiology (Eighth ed.). Philadelphia: Elsevier/Mosby. 2013. ISBN 978-0-323-10085-4.
- ↑ 2.0 2.1 "Cholecystokinin". Colorado State University. 28 January 2001. http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/gi/cck.html.
- ↑ Bernard, Claude (1856) (in French). Leçons de physiologie expérimentale appliquée à la médecine. 2. Paris, France: J.B. Baillière et fils. p. 430. https://www.biodiversitylibrary.org/item/16939#page/442/mode/1up. From p. 430: "En effet, si l'on ouvre le duodenum sur un animal vivant et que l'on touche l'orifice du conduit cholédoque avec une baguette de verre imprégnée d'acide acétique faible, on voit immédiatement un flot de bile lancé dans l'intestin; ce qui ne se fait pas si, au lieu de toucher l'orifice du conduit cholédoque avec un liquide acide, on le touche avec un liquide lègérement alcalin, comme du carbonate de soude par example." (Indeed, if one opens the duodenum on a living animal and touches the orifice of the bile duct with a glass rod impregnated with weak acetic acid, one immediately sees a stream of bile squirted into the intestine; which is not done if, instead of touching the orifice of the bile duct with an acidic liquid, it is touched with a slightly alkaline liquid, such as sodium carbonate for example.)
- ↑ Rehfeld, Jens F. (March 2021). "Cholecystokinin and the hormone concept". Endocrine Connections 10 (3): R139–R150. doi:10.1530/EC-21-0025. PMID 33640870.
- ↑ Wertheimer, E. (1903). "De l'action des acides et du chloral sur la sécrétion biliaire (d'après les expériences de M. Ch. Dubois)" (in French). Compte Rendus Hebdomadaires des Séances et Mémoires de la Société Biologie 55: 286–287. https://babel.hathitrust.org/cgi/pt?id=uc1.b4020783&view=1up&seq=310&skin=2021. From p. 287: "Ces expériences furent ensuite répétées après section préalable des pneumogastriques au cou et des sympathiques dans le thorax: cinq sur douze ont encore donné des résultats positifs." (These experiments [namely, introducing dilute acid into the duodenum in order to determine whether the acid then stimulated the secretion of bile] were then repeated after prior section [i.e., cutting] of the pneumogastric [i.e., vagus nerves] in the neck and the sympathetic [nerves] in the thorax: five out of twelve [experiments] again gave positive results.)
- ↑ Fleig, Charles (1904). "Du mode d'action des excitants chimiques des glandes digestives" (in French). Archives Internationales de Physiologie et de Biochimie 1: 286–346. https://babel.hathitrust.org/cgi/pt?id=uc1.b3888268&view=1up&seq=326&skin=2021. From pp. 316-317: "Expérience. — Chien 13 k. chloralosé. On isole une anse de duodéno-jejunum, … C'est là pour le moment une question non résolue et qui ne m'a donné aucun résultat." (Experiment: A dog of 13 kg. was anaesthetized with chloral hydrate. One isolates a section of the duodenum-jejunum; one introduces into it a solution of 0.5% HCl, and one collects the venous blood from the section as usual. Infusion of the [venous] blood which is administered during 30 minutes (about 100 cc.) to a dog of 7 kg. having a canula in the bile duct and [having] the cystic duct bound. The flow of bile is increased to double ([see] fig. 44). But is this humoral action due to the same secretin that acts on the pancrease or [is it due to the action of] a special "crinine" [i.e., a hypothetical hormone that's involved in digestion, like Fleig's "sapocrinine" (see p. 293)] on the liver? For the moment it's an unresolved question and [one] that has given me no result.)
- ↑ "A hormone mechanism for gall-bladder contraction and evacuation". American Journal of Physiology 86 (3): 599–613. October 1928. doi:10.1152/ajplegacy.1928.86.3.599.
- ↑ Harper, A. A.; Raper, H. S. (30 June 1943). "Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine". The Journal of Physiology 102 (1): 115–125. doi:10.1113/jphysiol.1943.sp004021. PMID 16991584.
- ↑ Jorpes, E.; Mutt, V. (Jan–Feb 1966). "Cholecystokinin and pancreozymin, one single hormone?". Acta Physiologica Scandinavica 66 (1): 196–202. doi:10.1111/j.1748-1716.1966.tb03185.x. PMID 5935672.
- ↑ "The history of gastrointestinal hormones and the Polish contribution to elucidation of their biology and relation to nervous system". Journal of Physiology and Pharmacology 54 (Suppl 3): 83–98. December 2003. PMID 15075466. http://www.jpp.krakow.pl/journal/archive/12_03_s3/pdf/83_12_03_s3_article.pdf.
- ↑ "Experiments with cholecystokinin in cholecystography". Acta Radiologica 49 (1): 25–30. January 1958. doi:10.3109/00016925809170975. PMID 13508336.
- ↑ Mutt, V.; Jorpes, J.E. (1968). "Structure of porcine cholecystokinin-pancreozymin. I. Cleavage with thrombin and with trypsin". European Journal of Biochemistry 6 (1): 156–162. doi:10.1111/j.1432-1033.1968.tb00433.x. PMID 5725809.
- ↑ 13.0 13.1 13.2 13.3 "Gastrointestinal hormones regulating appetite". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 361 (1471): 1187–209. July 2006. doi:10.1098/rstb.2006.1856. PMID 16815798.
- ↑ "Evidence that CCK-58 has structure that influences its biological activity". The American Journal of Physiology 270 (5 Pt 1): G860-8. May 1996. doi:10.1152/ajpgi.1996.270.5.G860. PMID 8967499.
- ↑ "Measurement of nonsulfated cholecystokinins". Scandinavian Journal of Clinical and Laboratory Investigation 74 (5): 424–31. August 2014. doi:10.3109/00365513.2014.900695. PMID 24734780.
- ↑ 16.0 16.1 "Genetic substrates of psychosis in patients with Parkinson's disease: A critical review". Journal of the Neurological Sciences 364: 33–41. May 2016. doi:10.1016/j.jns.2016.03.005. PMID 27084212.
- ↑ Ma, Yihe; Giardino, William J. (2022-09-01). "Neural circuit mechanisms of the cholecystokinin (CCK) neuropeptide system in addiction" (in en). Addiction Neuroscience 3: 100024. doi:10.1016/j.addicn.2022.100024. ISSN 2772-3925. PMID 35983578.
- ↑ Moran, T. H.; Schwartz, G. J. (1994). "Neurobiology of cholecystokinin". Critical Reviews in Neurobiology 9 (1): 1–28. ISSN 0892-0915. PMID 8828002. https://pubmed.ncbi.nlm.nih.gov/8828002.
- ↑ Ma, Yihe; Giardino, William J. (2022-09-01). "Neural circuit mechanisms of the cholecystokinin (CCK) neuropeptide system in addiction" (in en). Addiction Neuroscience 3: 100024. doi:10.1016/j.addicn.2022.100024. ISSN 2772-3925. PMID 35983578.
- ↑ "Neural hormonal regulation of exocrine pancreatic secretion". Pancreatology 1 (4): 320–35. 2001-01-01. doi:10.1159/000055831. PMID 12120211.
- ↑ 21.0 21.1 21.2 "Enteroendocrine hormones - central effects on behavior". Current Opinion in Pharmacology 13 (6): 977–82. December 2013. doi:10.1016/j.coph.2013.09.004. PMID 24091195.
- ↑ "Regulation of cholecystokinin secretion by intraluminal releasing factors". The American Journal of Physiology 269 (3 Pt 1): G319–27. September 1995. doi:10.1152/ajpgi.1995.269.3.G319. PMID 7573441.
- ↑ "Proglumide, a cholecystokinin antagonist, increases gastric emptying in rats". The American Journal of Physiology 252 (2 Pt 2): R353–60. February 1987. doi:10.1152/ajpregu.1987.252.2.R353. PMID 3812772.
- ↑ "Neural injury, repair, and adaptation in the GI tract. II. The elusive action of capsaicin on the vagus nerve". American Journal of Physiology. Gastrointestinal and Liver Physiology 275 (1): G8–G13. 1 July 1998. doi:10.1152/ajpgi.1998.275.1.G8. PMID 9655678.
- ↑ "CCK inhibits the orexigenic effect of peripheral ghrelin". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 288 (3): R751–8. March 2005. doi:10.1152/ajpregu.00094.2004. PMID 15550621.
- ↑ "Major biological actions of CCK--a critical evaluation of research findings". Experimental Brain Research 123 (1–2): 77–83. November 1998. doi:10.1007/s002210050546. PMID 9835394.
- ↑ 27.0 27.1 27.2 27.3 "Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y". Physiology & Behavior 107 (5): 699–710. December 2012. doi:10.1016/j.physbeh.2012.03.004. PMID 22429904.
- ↑ 28.0 28.1 "Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin". Depression and Anxiety 29 (9): 762–74. September 2012. doi:10.1002/da.21919. PMID 22553078.
- ↑ "Neurobiological investigations into the role of cholecystokinin in panic disorder". Journal of Psychiatry & Neuroscience 18 (4): 178–88. July 1993. PMID 8104032.
- ↑ "Distinct molecular mechanisms for agonist peptide binding to types A and B cholecystokinin receptors demonstrated using fluorescence spectroscopy". The Journal of Biological Chemistry 280 (2): 1044–50. January 2005. doi:10.1074/jbc.M409480200. PMID 15520004.
- ↑ "In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-B receptor imaging". Journal of Nuclear Medicine 45 (3): 485–94. March 2004. ProQuest 219229095. PMID 15001692. http://jnm.snmjournals.org/cgi/pmidlookup?view=long&pmid=15001692.
- ↑ "Identification of tyrosine 189 and asparagine 358 of the cholecystokinin 2 receptor in direct interaction with the crucial C-terminal amide of cholecystokinin by molecular modeling, site-directed mutagenesis, and structure/affinity studies". Molecular Pharmacology 63 (5): 973–82. May 2003. doi:10.1124/mol.63.5.973. PMID 12695525.
- ↑ "Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo". Molecular Biology of the Cell 19 (1): 198–206. January 2008. doi:10.1091/mbc.E07-05-0430. PMID 17978097.
- ↑ "Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor". The Journal of Neuroscience 25 (32): 7459–69. August 2005. doi:10.1523/JNEUROSCI.1193-05.2005. PMID 16093397.
- ↑ Kapas L (2010). Metabolic signals in sleep regulation: the role of cholecystokinin (PDF). The Journal of Neuroscience (PhD thesis). University of Szeged.
External links
- Cholecystokinin at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

