Physics:Cobalt oxide nanoparticle
| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
|
|
In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt(II,III) oxide Co3O4 of nanometer size, with various shapes and crystal structures.
Cobalt oxide nanoparticles have potential applications in lithium-ion batteries[1][2] and electronic gas sensors.[3][4]
Applications
Lithium-ion Battery
The cathodes of lithium-ion batteries are often made of lithiated oxides of cobalt, nickel, or manganese, that can readily and reversibly incorporate lithium ions in their molecular structure. Cobalt oxide nanomaterials, such as nanotubes,[1] offer high surface-to-volume ratio and short path lengths for lithium cation transport, leading to fast charging capabilities. However, capacity, coulombic efficiency, and cycle life may suffer due to excessive formation of SEI. The nanowires may incorporate other substances, for example, diphenylalanine.[5]
File:Cobale oxide graphene composite white background.tif Cobalt oxide particles may be anchored on substrates such as graphene to improve the dimensional stability of the anode and to prevent particle aggregation during lithium charge and discharge processes.[2]
Gas Sensor
Hollow nanospheres of cobalt oxide have been investigated as materials for gas sensor electrodes, for the detection of toluene, acetone, and other organic vapors.[3]
Cobalt oxide nanoparticles anchored on single-walled carbon nanotubes have been investigated for sensing nitrogen oxides NOx and hydrogen. This application takes advantage of the reactivity between the gas and the oxide, as well as the electrical connection with the substrate (both being p-type semiconductors). Nitrogen oxides react with the oxide as electron acceptors, reducing the electrode's resistance; whereas hydrogen acts as an electron donor, increasing the resistance.[4]
Medicine
Cobalt oxide nanoparticles have been observed to readily enter cells, a property that conceivably could lead to applications in hyperthermic treatment, gene therapy and drug delivery. However, their toxicity is an obstacle that would have to be overcome.[6]
Synthesis
Hydrothermal
Cobalt oxide is often obtained by hydrothermal synthesis in an autoclave.[7]
One-pot hydrothermal synthesis of metal oxide hollow spheres starts with carbohydrates and metal salts dissolved in water at 100-200 °C. The reaction produces carbon spheres, with metal ions integrated into the hydrophobic shell. The carbon cores are removed by calcination, leaving hollow metal oxide spheres. Surface area and thickness of the shell can be manipulated by varying the carbohydrate to metal salt concentration, as well as the temperature, pressure, and pH of the reaction medium, and the cations of the starting salts.[8] The completion time for the procedure varies from hours to days.[9]
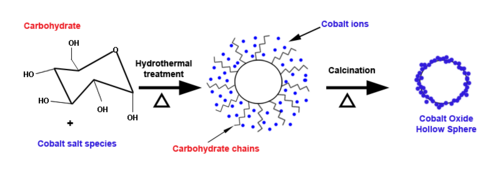
A drawback of this approach is its smaller yield compared to other methods.
Thermal decomposition
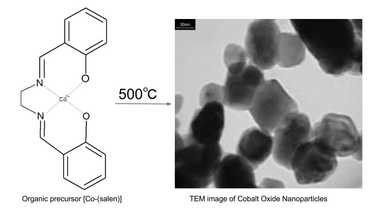
Another route to the synthesis of cobalt oxide nanoparticles is the thermal decomposition of organometallic compounds. For example, heating the metal salen complex bis(salicylaldehyde)ethylenediiminecobalt(II) ("Co-salen") in air to 500 °C.[10][11] The precursor Co-salen can be obtained by reacting cobalt(II) acetate tetrahydrate in propanol at 50 °C under nitrogen atmosphere with the salen ligand (bis(salicylaldehyde)ethylenediimine).[11]
From anchored precursors
Cobalt oxide/graphene composite are synthesized by first forming cobalt(II) hydroxide Co(OH)2 on the graphene sheet from a cobalt(II) salt and ammonium hydroxide NH4OH, which is then heated to 450 °C for two hours to yield the oxide.
Safety
Like most cobalt compounds, cobalt oxide nanoparticles are toxic to humans and also aquatic life.[12][13]
References
- ↑ 1.0 1.1 "Porous Co3O4 Nanotubes Derived From Co4(CO)12 Clusters on Carbon Nanotube Templates: A Highly Efficient Material For Li-Battery Applications". Advanced Materials 19 (24): 4505–4509. 17 December 2007. doi:10.1002/adma.200602513. Bibcode: 2007AdM....19.4505D.
- ↑ 2.0 2.1 "Graphene anchored with co(3)o(4) nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance". ACS Nano 4 (6): 3187–3194. June 2010. doi:10.1021/nn100740x. PMID 20455594.
- ↑ 3.0 3.1 "Solvothermal synthesis and gas-sensing performance of Co3O4 hollow nanospheres". Sensors and Actuators B: Chemical 136 (2): 494–498. March 2009. doi:10.1016/j.snb.2008.11.041.
- ↑ 4.0 4.1 "Nanocomposite of cobalt oxide nanocrystals and single-walled carbon nanotubes for a gas sensor application". Sensors and Actuators B: Chemical 150 (1): 160–166. September 2010. doi:10.1016/j.snb.2010.07.023.
- ↑ "Synthesis of diphenylalanine/cobalt oxide hybrid nanowires and their application to energy storage". ACS Nano 4 (1): 159–164. January 2010. doi:10.1021/nn901156w. PMID 20000841.
- ↑ "Engineered cobalt oxide nanoparticles readily enter cells". Toxicology Letters 189 (3): 253–259. September 2009. doi:10.1016/j.toxlet.2009.06.851. PMID 19539014.
- ↑ "Hydrothermal synthesis of transition metal oxides under mild conditions". Current Opinion in Solid State and Materials Science 1 (2): 227–232. April 1996. doi:10.1016/S1359-0286(96)80089-1. Bibcode: 1996COSSM...1..227W.
- ↑ "A Generalized Synthesis of Metal Oxide Hollow Spheres Using a Hydrothermal Approach". Chemistry of Materials 18 (16): 3808–3812. August 2006. doi:10.1021/cm052768u.
- ↑ "Magnetic nanoparticles: synthesis, protection, functionalization, and application". Angewandte Chemie 46 (8): 1222–1244. 12 February 2007. doi:10.1002/anie.200602866. PMID 17278160.
- ↑ "Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells". Materials Science and Engineering: B 193: 181–188. March 2015. doi:10.1016/j.mseb.2014.12.012.
- ↑ 11.0 11.1 "Synthesis and characterization of Co3O4 nanoparticles by a simple method". Comptes Rendus Chimie 17 (4): 352–358. April 2014. doi:10.1016/j.crci.2013.01.023. https://comptes-rendus.academie-sciences.fr/chimie/articles/10.1016/j.crci.2013.01.023/.
- ↑ "Quantitative Profiling of Protein S-Glutathionylation Reveals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress". ACS Nano 10 (1): 524–538. January 2016. doi:10.1021/acsnano.5b05524. PMID 26700264.
- ↑ "A Toxicological Profile for CobalT". Public Health Service, Agency for Toxic Substances and Disease Registry (U.S. Department of Health and Human Services). April 2004. https://www.atsdr.cdc.gov/toxprofiles/tp33.pdf.
 |
