Chemistry:β-Ketoisocaproic acid
From HandWiki
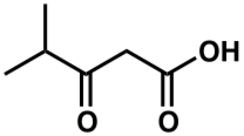
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methyl-3-oxopentanoic acid | |
| Other names
4-Methyl-3-oxopentanoic acid
4-Methyl-3-oxovaleric acid Isobutanoylacetic acid Isobutyrylacetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | Beta-ketoisocaproic+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.143 g·mol−1 |
| Density | 1.1 g cm−3 (at 20 °C) |
| Boiling point | 236 °C (457 °F; 509 K) ±23 at 760 mmHg |
| log P | 0.36 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
β-Ketoisocaproic acid, also known as 4-methyl-3-oxopentanoic acid, is an intermediate in the metabolism of leucine.[1][2] Its metabolic precursor and metabolic product in the leucine metabolic pathway are β-leucine and β-ketoisocaproyl-CoA, respectively.[1]
References
- ↑ 1.0 1.1 Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. May 2015. pp. 385–388, Figure 8.57. ISBN 978-0-12-387784-0. https://books.google.com/books?id=aTQTAAAAQBAJ. Retrieved 6 June 2016. "Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds ...
" - ↑ "Leucine metabolism". Technische Universität Braunschweig. http://www.brenda-enzymes.org/pathways/Maps/pathways/leucine_metabolism.svg.
 |

