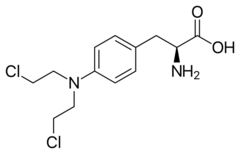
Chemistry:Melphalan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alkeran, Evomela, Phelinun, others |
| Other names | (2S)-2-amino-3-{4-[bis(2-chloroethyl)amino]phenyl}propanoic acid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682220 |
| License data | |
| Routes of administration | By mouth, intravenous, intra-arterial |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 25–89% (By mouth) |
| Metabolism | Hydrolysis to inactive metabolites |
| Elimination half-life | 1.5 ± 0.8 hours |
| Excretion | Kidney (IV: 5.8–21.3%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H18Cl2N2O2 |
| Molar mass | 305.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Melphalan, sold under the brand name Alkeran among others, is a chemotherapy medication used to treat multiple myeloma; malignant lymphoma; lymphoblastic and myeloblastic leukemia; childhood neuroblastoma; ovarian cancer; mammary adenocarcinoma; and uveal melanoma.[1][2][4][5] It is taken by mouth or by injection into a vein.[5]
Common side effects include nausea and bone marrow suppression.[5] Other severe side effects may include anaphylaxis and the development of other cancers.[5] Use during pregnancy may result in harm to the fetus.[6] Melphalan belongs to the class of nitrogen mustard alkylating agents.[5] It works by interfering with the creation of DNA and RNA.[5]
Melphalan was approved for medical use in the United States in 1964.[5] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[8]
Medical uses
In the European Union, melphalan is indicated for the treatment of multiple myeloma; malignant lymphoma (Hodgkin, non-Hodgkin lymphoma); acute lymphoblastic and myeloblastic leukemia; childhood neuroblastoma; ovarian cancer; and mammary adenocarcinoma.[4]
In the United States, melphalan is used as a high-dose conditioning treatment prior to hematopoietic progenitor (stem) cell transplantation in people with multiple myeloma.[2][9] In the European Union, it is indicated, in combination with other cytotoxic medicinal products, as reduced intensity conditioning treatment prior to allogeneic haematopoietic stem cell transplantation in malignant haematological diseases in adults.[4]
In August 2023, the US Food and Drug Administration approved melphalan (Hepzato) as a liver-directed treatment for adults with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation.[10][11]
Side effects
Common side effects include:[5]
- Nausea
- Bone marrow suppression, including
- Decreased white blood cell count causing increased risk of infection
- Decreased platelet count causing increased risk of bleeding
Less common side effects include:
- Severe allergic reactions[5]
- Pulmonary fibrosis (scarring of lung tissue) including fatal outcomes (usually only with prolonged use)
- Hair loss
- Interstitial pneumonitis
- Rash
- Itching
- Irreversible bone marrow failure due to melphalan not being withdrawn early enough
- Cardiac arrest
Mechanism of action
Melphalan chemically alters the DNA nucleotide guanine through alkylation, and causes linkages between strands of DNA. This chemical alteration inhibits DNA synthesis and RNA synthesis, functions necessary for cells to survive. These changes cause cytotoxicity in both dividing and non-dividing tumor cells.[12]
Synthesis
4-Nitro-L-phenylalanine (1) was converted to its phthalimide by heating with phthalic anhydride, and this was converted to its ethyl ester (2). Catalytic hydrogenation produced the corresponding aniline. Heating in acid with oxirane, followed by treatment with phosphorus oxychloride provided the bischloride, and removal of the protecting groups by heating in hydrochloric acid gave melphalan (3).
Society and culture
Legal status
On 17 September 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for melphalan.[16] The applicant for this medicinal product is Adienne S.r.l. S.U.[16] Melphalan was approved for medical use in the European Union in November 2020.[4]
References
- ↑ 1.0 1.1 "Alkeran- melphalan tablet, film coated". 18 November 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ff913271-0090-4832-a0fe-5154fe8f97b9.
- ↑ 2.0 2.1 2.2 "Evomela- melphalan injection, powder, lyophilized, for solution". 31 December 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c5d68b96-14bd-4605-b6d2-2bf8b0c5ca8a.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/201848s000lbl.pdf
- ↑ 4.0 4.1 4.2 4.3 4.4 "Phelinun EPAR". 15 September 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/phelinun. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Melphalan Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/melphalan.html.
- ↑ "Melphalan Use During Pregnancy". https://www.drugs.com/pregnancy/melphalan.html.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 873–874. ISBN 9780857113382.
- ↑ "Evomela (Captisol-enabled melphalan HCl) for Injection". 30 November 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/207155Orig1s000_Orig2s000TOC.cfm.
- ↑ "Oncology (Cancer) / Hematologic Malignancies Approval Notifications". 14 August 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Delcath Systems, Inc. Announces FDA Approval of Hepzato Kit for the Treatment of Adult Patients with Unresectable Hepatic-Dominant Metastatic Uveal Melanoma" (Press release). Delcath Systems. 14 August 2023. Retrieved 7 September 2023 – via PR Newswire.
- ↑ "Melphalan". National Cancer Institute. http://www.cancer.gov/drugdictionary?cdrid=42973.
- ↑ "Cyto-active amino-acid and peptide derivatives. Part I. Substituted phenylalanines". Journal of the Chemical Society (Resumed): 2409. 1954. doi:10.1039/JR9540002409.
- ↑ "Cyto-active amino-acids and peptides. Part II. Resolution of para-substituted phenylalanines and synthesis of p-di-(2-chloroethyl)amino-DL-phenyl[?-14C]alanine". Journal of the Chemical Society (Resumed): 1223–1230. 1955. doi:10.1039/JR9550001223.
- ↑ "Studies on the anti-tumour activity of p-di-(2-chloroethyl) aminophenylalanine (sarcolysine)". Lancet 266 (6882): 169–71. 1955. doi:10.1016/S0140-6736(55)92736-7. PMID 13243678.
- ↑ 16.0 16.1 "Phelinun: Pending EC decision". 18 September 2020. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/phelinun. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
 |


