Chemistry:Berkelium(III) fluoride
From HandWiki
| |
| Names | |
|---|---|
| Other names
berkelium trifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| BkF3 | |
| Molar mass | 304 g·mol−1 |
| Appearance | yellow-green solid |
| Density | 9.70 g/cm3 |
| Related compounds | |
Related compounds
|
Berkelium tetrafluoride Einsteinium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Berkelium(III) fluoride is a binary inorganic compound of berkelium and fluorine with the chemical formula BkF3.[1][2][3]
Synthesis
The compound can be prepared by treating Bk2O3 with a gaseous mixture of H2 and HF at 600 °C.[4]
Physical properties
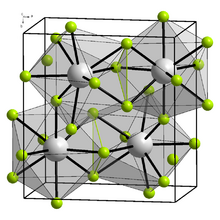
Berkelium trifluoride forms a yellow-green solid with two structures.[5] At low temperature, it is orthorhombic (YF3 structure), with lattice parameters a = 670 pm, b = 709 pm, and c = 441 pm. At high temperature, it is trigonal (LaF3 structure), with lattice parameters a = 697 pm and c = 714 pm. The transition temperature of BkF3 is between 350 and 600 °C.[6][7]
Chemical properties
Berkelium trifluoride is reduced by lithium to obtain metallic berkelium:
- BkF
3 + 3Li → Bk + 3LiF
- BkF
References
- ↑ Peterson, J. R.; Cunningham, B. B. (1 August 1968). "Crystal structures and lattice parameters of the compounds of berkelium—IV berkelium trifluoride" (in en). Journal of Inorganic and Nuclear Chemistry 30 (7): 1775–1784. doi:10.1016/0022-1902(68)80353-7. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190268803537. Retrieved 11 April 2023.
- ↑ Edelstein, Norman M. (11 September 2013) (in en). Actinides in Perspective: Proceedings of the Actinides—1981 Conference, Pacific Grove, California, USA, 10-15 September 1981. Elsevier. p. 334. ISBN 978-1-4831-9051-8. https://books.google.com/books?id=hlz-BAAAQBAJ&dq=berkelium+trifluoride&pg=PA334. Retrieved 11 April 2023.
- ↑ "WebElements Periodic Table » Berkelium » berkelium trifluoride". webelements.com. https://www.webelements.com/compounds/berkelium/berkelium_trifluoride.html.
- ↑ Mi͡asoedov, Boris Fedorovich (1974) (in en). Analytical Chemistry of Transplutonium Elements. Wiley. p. 97. ISBN 978-0-470-62715-0. https://books.google.com/books?id=_fuFAAAAIAAJ&q=berkelium+trifluoride. Retrieved 11 April 2023.
- ↑ Ahrland, S.; Bagnall, K. W.; Brown, D. (7 June 2016) (in en). The Chemistry of the Actinides: Comprehensive Inorganic Chemistry. Elsevier. p. 161. ISBN 978-1-4831-5934-8. https://books.google.com/books?id=awRPDAAAQBAJ&dq=berkelium+trifluoride&pg=PA161. Retrieved 11 April 2023.
- ↑ Peterson, J. R.; Fahey, J. A.; Baybarz, R. D. (1 October 1971). "The crystal structures and lattice parameters of berkelium metal" (in en). Journal of Inorganic and Nuclear Chemistry 33 (10): 3345–3351. doi:10.1016/0022-1902(71)80656-5. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190271806565. Retrieved 11 April 2023.
- ↑ Ensor, D. D.; Peterson, J. R.; Haire, R. G.; Young, J. P. (1 January 1981). "Absorption spectrophotometric study of berkelium(III) and (IV) fluorides in the solid state" (in en). Journal of Inorganic and Nuclear Chemistry 43 (5): 1001–1003. doi:10.1016/0022-1902(81)80164-9. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190281801649. Retrieved 11 April 2023.
 |

