Chemistry:Affinine
From HandWiki
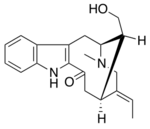
| |
| Names | |
|---|---|
| IUPAC name
(2S,6R,14S,E)-5-ethylidene-14-(hydroxymethyl)-3,14-dimethyl-2,3,4,5,6,7-hexahydro-1H-2,6-methanoazecino[5,4-b]indol-8(9H)-one
| |
| Other names
17-hydroxy-vobasan-3-one,
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H24N2O2 | |
| Molar mass | 324.424 g·mol−1 |
| Melting point | 265°C (decomp.) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Affinine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana.[1][2] Structurally it can be considered a member of the vobasine alkaloid family and may be synthesized from tryptophan.[3] Limited pharmacological testing has indicated that it may be an effective inhibitor of both acetylcholinesterase and butyrylcholinesterase.[4]
See also
References
- ↑ 1.0 1.1 Weisbach, Jerry A.; Raffauf, Robert F.; Ribeiro, Oscar; Macko, Edward; Douglas, Bryce (April 1963). "Problems in chemotaxonomy I. Alkaloids ofPeschiera affinis". Journal of Pharmaceutical Sciences 52 (4): 350–353. doi:10.1002/jps.2600520408. PMID 13999677.
- ↑ Monnerat, Cecilia Silva; Souza, Jucimar Jorgeane de; Mathias, Leda; Braz-Filho, Raimundo; Vieira, Ivo José C. (November 2005). "A new indole alkaloid isolated from Tabernaemontana hystrix steud (Apocynaceae)". Journal of the Brazilian Chemical Society 16 (6b): 1331–1335. doi:10.1590/S0103-50532005000800004.
- ↑ Yang, Jie; Rallapalli, Sundari K.; Cook, James M. (February 2010). "The first enantiospecific total synthesis of the 3-oxygenated sarpagine indole alkaloids affinine and 16-epiaffinine, as well as vobasinediol and 16-epivobasinediol". Tetrahedron Letters 51 (5): 815–817. doi:10.1016/j.tetlet.2009.12.002.
- ↑ Vieira, IJ; Medeiros, WL; Monnerat, CS; Souza, JJ; Mathias, L; Braz-Filho, R; Pinto, AC; Sousa, PM et al. (September 2008). "Two fast screening methods (GC-MS and TLC-ChEI assay) for rapid evaluation of potential anticholinesterasic indole alkaloids in complex mixtures.". Anais da Academia Brasileira de Ciências 80 (3): 419–26. doi:10.1590/S0001-37652008000300003. PMID 18797794.
 |
