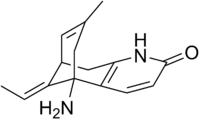
Chemistry:Huperzine A
 | |
 | |
| Clinical data | |
|---|---|
| Other names | HupA |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 10-14h[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H18N2O |
| Molar mass | 242.322 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 217 to 219 °C (423 to 426 °F) |
| |
| |
| | |
Huperzine A is a naturally-occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata[2] and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian.[3] Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a 2013 meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution.[4][5] Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine (ACh) by the enzyme acetylcholinesterase. It is commonly available over the counter as a nutritional supplement and marketed as a memory and concentration enhancer.
Pharmacological effects
Huperzine A is extracted from Huperzia serrata.[2] It is a reversible acetylcholinesterase inhibitor[6][7][8][9] and NMDA receptor antagonist[10] that crosses the blood–brain barrier.[11] Acetylcholinesterase is an enzyme that catalyzes the breakdown of the neurotransmitter ACh and other choline esters that function as neurotransmitters. The structure of the complex of huperzine A with acetylcholinesterase has been determined by X-ray crystallography (PDB code: 1VOT; see the 3D structure).[12]
Huperzine A has been investigated as a possible treatment for diseases characterized by neurodegeneration such as Alzheimer's disease,[2][13] and there is some evidence from small-scale studies that it can benefit cognitive functioning, global clinical status, and ability to engage in activities of daily living (ADLs) among individuals with the disease. In a 2016 systematic review of systematic reviews,[14] huperzine A was associated with a standardized mean difference of 1.48 (95% CI, 0.95-2.02) compared to placebo on measures of ADL among people with dementia, but the evidence was very low-quality and uncertain. In a 2022 umbrella review,[15] huperzine A was associated with broad benefits to dementia patients' cognitive functioning, but the degree of heterogeneity in measurements and outcomes of the reviewed studies indicated publication bias toward huperzine A benefit.
Adverse effects
Huperzine A may present with mild cholinergic side effects such as nausea, vomiting, and diarrhea.[5] Slight muscle twitching and slurred speech might also occur, as well as excessive saliva excretion and sweating. The use of huperzine A during pregnancy and lactation is not recommended due to the lack of sufficient safety data.[16]
Drug interactions
Huperzine A may have additive effects if taken with drugs causing bradycardia, such as beta-blockers,[17] which may decrease heart rate. Theoretically, there may be possible additive cholinergic effects if huperzine A is taken with other acetylcholinesterase inhibitors or cholinergic agents.[18]
Safety
Huperzine A, in spite of the possible cholinergic side effects, seems to have a wide margin of safety. Toxicology studies show huperzine A to be non-toxic even when administered at 50-100 times the human therapeutic dose. The extract is active for 6 hours at a dose of 2 μg/kg with no remarkable side effects.[19]
Other possible uses
Huperzine A might be useful in the treatment of organophosphate nerve agent poisoning by preventing damage to the central nervous system caused by such agents. [20] [21]
Synthesis
Two scalable and efficient total syntheses of huperzine A have been reported.[22][23]
References
- ↑ "Pharmacokinetics of huperzine A following oral administration to human volunteers". European Journal of Drug Metabolism and Pharmacokinetics 32 (4): 183–187. 2007. doi:10.1007/BF03191002. PMID 18348466.
- ↑ 2.0 2.1 2.2 "The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease". Pharmacology, Biochemistry, and Behavior 75 (3): 675–686. June 2003. doi:10.1016/S0091-3057(03)00111-4. PMID 12895686.
- ↑ "Huperzine alkaloids from Australasian and southeast Asian Huperzia". Pharmaceutical Biology 48 (9): 1073–1078. September 2010. doi:10.3109/13880209.2010.485619. PMID 20731560.
- ↑ "Huperzine A for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials". PLOS ONE 8 (9): e74916. 2013. doi:10.1371/journal.pone.0074916. PMID 24086396. Bibcode: 2013PLoSO...874916Y.
- ↑ 5.0 5.1 "Huperzine A for Alzheimer's disease". The Cochrane Database of Systematic Reviews CD005592 (2): CD005592. April 2008. doi:10.1002/14651858.CD005592.pub2. PMID 18425924.
- ↑ "Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine". Acta Pharmacologica Sinica 27 (1): 1–26. January 2006. doi:10.1111/j.1745-7254.2006.00255.x. PMID 16364207. "Huperzine A (HupA), a novel alkaloid isolated from the Chinese herb Huperzia serrata, is a potent, highly specific and reversible inhibitor of acetylcholinesterase (AChE).".
- ↑ Herbs and Nutrients for the Mind: A Guide to Natural Brain Enhancers. Greenwood Publishing Group. 2004. pp. 191. ISBN 978-0275983949. https://books.google.com/books?id=a5AMBY9ekzcC&q=Huperzine&pg=PA191.
- ↑ "Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis". Journal of Neural Transmission 116 (4): 457–465. April 2009. doi:10.1007/s00702-009-0189-x. PMID 19221692.
- ↑ "Huperzine A: A novel acetylcholinesterase inhibitor". Drugs of the Future 24 (6): 647. 1999. doi:10.1358/dof.1999.024.06.545143.
- ↑ "[+]-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats". Chemico-Biological Interactions 175 (1–3): 387–395. September 2008. doi:10.1016/j.cbi.2008.05.023. PMID 18588864.
- ↑ "Huperzine A--an interesting anticholinesterase compound from the Chinese herbal medicine". Acta Medica 41 (4): 155–157. 1998. doi:10.14712/18059694.2019.181. PMID 9951045.
- ↑ "Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A". Nature Structural Biology 4 (1): 57–63. January 1997. doi:10.1038/nsb0197-57. PMID 8989325.
- ↑ "Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease". Current Medicinal Chemistry 7 (3): 355–374. March 2000. doi:10.2174/0929867003375281. PMID 10637369.
- ↑ Laver, Kate; Dyer, Suzanne; Whitehead, Craig; Clemson, Lindy; Crotty, Maria (2016-04-27). "Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews". BMJ Open 6 (4): e010767. doi:10.1136/bmjopen-2015-010767. ISSN 2044-6055. PMID 27121704. PMC 4854009. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854009/.
- ↑ Fan, Fangcheng; Liu, Hua; Shi, Xiaojie; Ai, Yangwen; Liu, Qingshan; Cheng, Yong (2022-02-01). "The Efficacy and Safety of Alzheimer’s Disease Therapies: An Updated Umbrella Review". Journal of Alzheimer's Disease 85 (3): 1195–1204. doi:10.3233/JAD-215423. https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-215423.
- ↑ "Huperzine A". Natural Standard. https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=764#safety.
- ↑ "Huperzine A". American Journal of Health-System Pharmacy 57 (6): 530, 533-530, 534. March 2000. doi:10.1093/ajhp/57.6.530. PMID 10754762.
- ↑ "Old Chinese herbal medicine used for fever yields possible new Alzheimer disease therapy". JAMA 277 (10): 776. March 1997. doi:10.1001/jama.1997.03540340010004. PMID 9052690.
- ↑ "Review of the value of huperzine as pretreatment of organophosphate poisoning". Neurotoxicology 23 (1): 1–5. May 2002. doi:10.1016/S0161-813X(02)00015-3. PMID 12164543.
- ↑ "Review of the Value of Huperzine as Pretreatment of Organophosphate Poisoning". 22 April 2013. https://nutritionreview.org/2013/04/huperzinea/.
- ↑ "[Advances on study of organophosphate poisoning prevented by Huperzine A"]. Wei Sheng Yan Jiu = Journal of Hygiene Research 34 (2): 224–226. March 2005. PMID 15952670. https://europepmc.org/article/med/15952670.
- ↑ "A robust and scalable synthesis of the potent neuroprotective agent (−)-huperzine A". Chemical Science 2 (11): 2251–2253. 2011. doi:10.1039/C1SC00455G.
- ↑ "Development of a Large-Scale Synthetic Route to Manufacture (−)-Huperzine A". Organic Process Research & Development 16 (4): 635–642. 2012. doi:10.1021/op200360b.
External links
 |
