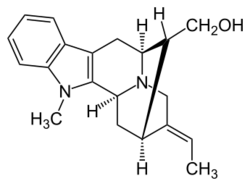
Chemistry:Affinisine
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
1-Methylsarpagan-17-ol
| |
| Systematic IUPAC name
[(6S,9E,10R,11R,12S)-9-Ethylidene-5-methyl-5,6,7,8,9,10,11,11a,12-decahydro-6,10-methanoindolo[2,3-g]quinolizin-11-yl]methanol | |
| Other names
De(hydroxymethyl)voachalotinol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H24N2O | |
| Molar mass | 308.425 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana.[1][2] Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.[3][4]
Pharmacology
Limited pharmacological testing has indicated that affinisine may effectively inhibit acetylcholinesterase and butyrylcholinesterase.[1][5]
See also
References
- ↑ 1.0 1.1 Andrade, Marcelo T.; Lima, Josélia A.; Pinto, Angelo C.; Rezende, Claudia M.; Carvalho, Meriane P.; Epifanio, Rosângela A. (June 2005). "Indole alkaloids from Tabernaemontana australis (Müell. Arg) Miers that inhibit acetylcholinesterase enzyme". Bioorganic & Medicinal Chemistry 13 (12): 4092–4095. doi:10.1016/j.bmc.2005.03.045. PMID 15911323.
- ↑ Clivio, Pascale; Richard, Bernard; Deverre, Jean-Robert; Sevenet, Thierry; Zeches, Monique; Le Men-Oliver, Louisette (January 1991). "Alkaloids from leaves and root bark ofErvatamia hirta". Phytochemistry 30 (11): 3785–3792. doi:10.1016/0031-9422(91)80111-D.
- ↑ Liu, Xiaoxiang; Wang, Tao; Xu, Qingge; Ma, Chunrong; Cook, James M (August 2000). "Enantiospecific total synthesis of the enantiomer of the indole alkaloid affinisine". Tetrahedron Letters 41 (33): 6299–6303. doi:10.1016/S0040-4039(00)01061-3.
- ↑ Liao, Xuebin; Zhou, Hao; Yu, Jianming; Cook, James M. (November 2006). "An Improved Total Synthesis of (+)-Macroline and Alstonerine as Well as the Formal Total Synthesis of (−)-Talcarpine and (−)-Anhydromacrosalhine−methine". The Journal of Organic Chemistry 71 (23): 8884–8890. doi:10.1021/jo061652u. PMID 17081019.
- ↑ Vieira, Ivo J.C.; Medeiros, Walter L.B.; Monnerat, Cecilia S.; Souza, Jucimar J.; Mathias, Leda; Braz-Filho, Raimundo; Pinto, Angelo C.; Sousa, Priscila M. et al. (September 2008). "Two fast screening methods (GC-MS and TLC-ChEI assay) for rapid evaluation of potential anticholinesterasic indole alkaloids in complex mixtures". Anais da Academia Brasileira de Ciências 80 (3): 419–426. doi:10.1590/S0001-37652008000300003. PMID 18797794.
 |
