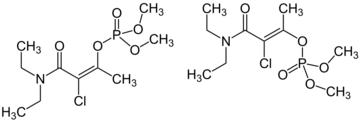
Chemistry:Phosphamidon
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
(E/Z)-[3-Chloro-4-(diethylamino)-4-oxobut-2-en-2-yl] dimethyl phosphate
| |
| Other names
Dimecron
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C10H19ClNO5P | |
| Molar mass | 299.69 g·mol−1 |
| Density | 1.2132 g/cm3[1] |
| Melting point | 120 to 123 °C (248 to 253 °F; 393 to 396 K)[3] |
| Boiling point | 162 °C (324 °F; 435 K) (1.5 mmHg)[2] |
| Miscible | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
13 mg/kg (mouse, oral)[3] 6 mg/kg (mouse, IV)[3] 20 mg/kg (rat, oral)[3] 26 mg/kg (rat, subcut.)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Phosphamidon is an organophosphate insecticide first reported in 1960.[3][2] It acts as a cholinesterase inhibitor.
The commercial product typically exists as a mixture of 70% (Z)-isomer and 30% (E)-isomer.[1]
Toxicity and regulation
Phosphamidon is very highly toxic to mammals and is listed as WHO Hazard Class Ia.[1] A harvester developed symptoms of moderately severe poisoning after working in a field that had been sprayed with the chemical 2 weeks earlier. He collapsed and exhibited significant depression of serum cholinesterase, but recovered completely within 2 days after successful treatment with atropine.[4] International trade of phosphamidon is covered by the Rotterdam Convention.
References
- ↑ 1.0 1.1 1.2 Data Sheet on Pesticides No. 74: Phosphamidon, International Programme on Chemical Safety
- ↑ 2.0 2.1 Bachmann, Fritz (1960). "Phosphamidon, a new phosphate ester with systemic action". Proc. Intern. Cong. Crop. Protection, 4th Congr., Hamburg 2: P1153-1155.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Jacques, R.; Bein, H. J. (1960). "Toxicology and pharmacology of a new systemic phosphoric acid ester insecticide phosphamidon (2-chloro-2-diethylcarbamoyl-1-methylvinyl dimethyl phosphate)". Archiv für Toxikologie 18: 316–330. doi:10.1007/BF02226232.
- ↑ S. Gitelson, J. T. Davidson, A. Werczberger. Phosphamidon poisoning. Br. J. Ind. Med. 22: 236-239, 1965.
 |

