Chemistry:Acotiamide
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Acofide |
| Other names | YM-443, Z-338 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 84.21–85.95% |
| Metabolism | UGT1A8 and 1A9 (major) |
| Elimination half-life | 10.9–21.7 hours |
| Excretion | Feces (92.7%), urine (5.3%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
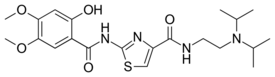
| Formula | C21H30N4O5S |
| Molar mass | 450.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Acotiamide, sold under the brand name Acofide,[2][3] is a medication manufactured and approved in Japan for the treatment of postprandial fullness, upper abdominal bloating, and early satiation due to functional dyspepsia.[4] It acts as an acetylcholinesterase inhibitor.
References
- ↑ "Acofide (acotiamide hydrochloride hydrate) Tablets Review Report". https://www.pmda.go.jp/files/000153467.pdf.
- ↑ "Acotiamide: first global approval". Drugs 73 (12): 1377–83. August 2013. doi:10.1007/s40265-013-0100-9. PMID 23881665.
- ↑ "[Pharmacological and clinical profile of acotiamide hydrochloride hydrate (Acofide(®) Tablets 100 mg), a novel therapeutic agent for functional dyspepsia (FD)]" (in Japanese). Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica 143 (2): 84–94. February 2014. doi:10.1254/fpj.143.84. PMID 24531902.
- ↑ "Clinical trial: dose-dependent therapeutic efficacy of acotiamide hydrochloride (Z-338) in patients with functional dyspepsia - 100 mg t.i.d. is an optimal dosage". Neurogastroenterology and Motility 22 (6): 618–e173. June 2010. doi:10.1111/j.1365-2982.2009.01449.x. PMID 20059698.
 |
