Chemistry:Trichloronate
From HandWiki
| |
| Names | |
|---|---|
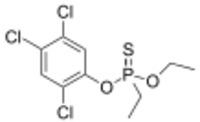
| Preferred IUPAC name
O-Ethyl O-(2,4,5-trichlorophenyl) ethylphosphonothioate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H12Cl3O2PS | |
| Molar mass | 333.59 g·mol−1 |
| Appearance | Amber colored odorless liquid[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Trichloronate is a highly toxic organophosphate insecticide.[2] It is used against vegetable fly larvae and soil pests.[1]
Case reports indicate exposure to the chemical can cause fatal encephalopathy.[3] Its aquatic toxicity has been measured at significantly higher against Ceriodaphnia dubia and Daphnia magna.[4]
References
- ↑ 1.0 1.1 "Hazardous Substance Fact Sheet: Trichloronate". June 1999. http://nj.gov/health/eoh/rtkweb/documents/fs/2837.pdf.
- ↑ Trichloronate at cameochemicals.noaa.gov.
- ↑ "Fatal encephalopathy in acute poisoning with organophosphorus insecticides. A clinico-pathologic study of two cases". Clin Neurol Neurosurg 81 (4): 247–54. 1979. doi:10.1016/0303-8467(79)90029-5. PMID 233207.
- ↑ "Separation and aquatic toxicity of enantiomers of the organophosphorus insecticide trichloronate". Chirality 18 (9): 713–6. 2006. doi:10.1002/chir.20323. PMID 16845672.
 |
