Chemistry:Conodurine
From HandWiki
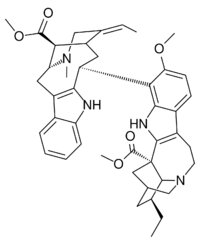
| |
| Names | |
|---|---|
| IUPAC name
methyl 17-ethyl-5-[(15Z)-15-ethylidene-18-methoxycarbonyl-17-methyl-10,17-diazatetracyclo[12.3.1.03,11.04,9]octadeca-3(11),4,6,8-tetraen-12-yl]-6-methoxy-3,13-diazapentacyclo[13.3.1.02,10.04,9.013,18]nonadeca-2(10),4(9),5,7-tetraene-1-carboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C43H52N4O5 | |
| Molar mass | 704.912 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Conodurine is an acetylcholinesterase inhibitor and butyrylcholinesterase inhibitor isolated from Tabernaemontana.[1][2][3]
See also
- Conolidine
- Confoline
References
- ↑ Vieira, IJ; Medeiros, WL; Monnerat, CS; Souza, JJ; Mathias, L; Braz-Filho, R; Pinto, AC; Sousa, PM et al. (Sep 2008). "Two fast screening methods (GC-MS and TLC-ChEI assay) for rapid evaluation of potential anticholinesterasic indole alkaloids in complex mixtures". An. Acad. Bras. Ciênc. 80 (3): 419–26. doi:10.1590/s0001-37652008000300003. PMID 18797794.
- ↑ "Annals of the Brazilian Academy of Sciences". https://www.scielo.br/pdf/aabc/v80n3/a03v80n3.pdf.
- ↑ "Conodurine, conoduramine, and ervahanine derivatives from Tabernaemontana corymbosa". Phytochemistry 63 (5): 625–9. July 2003. doi:10.1016/s0031-9422(03)00087-6. PMID 12809725.
 |
