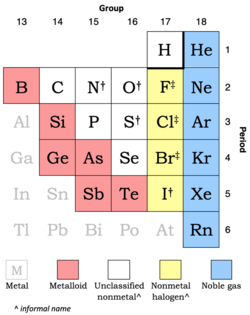
Chemistry:Nonmetal
Extract of periodic table showing how often each element is classified as a nonmetal:
14 effectively always[n 1] 3 frequently[n 2] 6 sometimes (metalloids)[n 3]
Nearby metals are shown in a gray font.[n 4]
There is no precise definition of a nonmetal; which elements are counted as such varies.
Hydrogen is usually in group 1 (as in the full table below) but can be in group 17 (as in the extract above).[n 5]
| Template:Lime▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ Template:Lime ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ Template:YellowTemplate:Lime ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ Template:YellowTemplate:Lime ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ Template:YellowTemplate:Lime ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ Template:Lime ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ Template:Lime ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ |
| ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ |
A nonmetal is a chemical element that, in the broadest sense of the term, has a relatively low density and high electronegativity; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). They are usually poor conductors of heat and electricity, and brittle or crumbly when solid due to their electrons having low mobility. In contrast, metals are good conductors and most are easily flattened into sheets and drawn into wires since their electrons are generally free-moving. Nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds.
Two nonmetals, hydrogen and helium, make up about 99% of ordinary matter in the observable universe by mass. Five nonmetallic elements, hydrogen, carbon, nitrogen, oxygen and silicon, make up most of the Earth's crust, atmosphere, oceans and biosphere.
The distinctive properties of nonmetallic elements allow for specific applications that often cannot be fulfilled by metallic elements alone. Living organisms are composed almost entirely of the nonmetals hydrogen, oxygen, carbon, and nitrogen. Nonmetallic elements are important to industries ranging from electronics and energy storage to agriculture and chemical production.
While the term non-metallic dates from as far back as 1566, there is no widely agreed precise definition of a nonmetal. Some elements have a marked mixture of metallic and nonmetallic properties, and which of these borderline cases are counted as nonmetals varies depending on the classification criteria used. Generally, from 14 to 23 elements are recognized as nonmetals.
Definition and applicable elements
A nonmetal is a chemical element that, in the broadest sense of the term, has a relatively low density and high electronegativity.[7] More generally they are deemed to lack a preponderance of metallic properties such as luster or shininess; the capacity to be flattened into a sheet or drawn into a wire; good thermal and electrical conductivity; and the capacity to form a basic (rather than acidic) oxide.[8] Since there is no rigorous definition of a nonmetal,[9] some variation exists among sources as to which elements are classified as such. The decisions involved depend on which property or properties are regarded as most indicative of nonmetallic or metallic character.[10][n 6]
Although Steudel,[11][n 7] in 2020, recognised twenty-three elements as nonmetals, any such list is open to challenge.[1] The fourteen elements that are almost always recognized as nonmetals are hydrogen, oxygen, nitrogen, and sulfur; the highly reactive halogens fluorine, chlorine, bromine, and iodine; and the noble gases helium, neon, argon, krypton, xenon, and radon, as listed in Hawley’s Condensed Chemical Dictionary.[1] While carbon, phosphorus and selenium were included as nonmetals, it had earlier been reported that these three elements were instead sometimes counted as metalloids.[2] The elements commonly recognized as metalloids (boron; silicon and germanium; arsenic and antimony; and tellurium) are sometimes counted as an intermediate class between the metals and the nonmetals when the criteria used to distinguish between metals and nonmetals are inconclusive.[12] At other times they are counted as nonmetals in light of their predominately nonmetallic (weakly acidic) chemistry.[13]
Of the 118 known elements,[14] no more than about 20% are regarded as nonmetals.[15] The status of a few elements is less certain. Astatine, the fifth halogen, is often ignored on account of its rarity and intense radioactivity;[16] theory and experimental evidence suggest it is a metal.[17] The superheavy elements copernicium (element 112), flerovium (114), and oganesson (118) may turn out to be nonmetals. (As of April 2023) their status has not been confirmed.[18]
General properties
- Properties noted in this section refer to the elements in their most stable forms in ambient conditions
Physical
of some nonmetallic elements
About half of nonmetallic elements are gases; most of the rest are shiny solids. Bromine, the only liquid, is so volatile that it is usually topped by a layer of its fumes; sulfur is the only colored solid nonmetal.[n 8] The fluid nonmetals have very low densities, melting points, and boiling points, and are poor conductors of heat and electricity.[21] The solid elements have low densities, are brittle or crumbly with low mechanical and structural strength,[22] and are poor to good conductors.[n 9]
The physical differences between metals and nonmetals arise from internal and external atomic forces. Internally, the positive charge arising from the protons in an atom's nucleus acts to hold the atom's outer electrons in place. Externally, the same electrons are subject to attractive forces from the protons in nearby atoms. When the external forces are greater than, or equal to, the internal force, the outer electrons are expected to become free to move between atoms, and metallic properties are predicted. Otherwise nonmetallic properties are expected.[26]
Those nonmetals existing as discrete atoms (xenon, for example) or molecules (oxygen, sulfur, and bromine, for example) have low melting and boiling points, and many are gases at room temperature, since they are held together by weak London dispersion forces acting between their atoms or molecules.[27] Nonmetals that form giant structures, such as chains of up to 1,000 atoms (selenium),[28] sheets (carbon as graphite, for example),[29] or three-dimensional lattices (silicon, for example),[30] have higher melting and boiling points, and are all solids, as it takes more energy to overcome their stronger covalent bonds.[31] Those closer to the left side of the periodic table, or further down a column, often have some weak metallic interactions between their molecules, chains, or layers, consistent with their proximity to the metals; this occurs in boron,[32] carbon,[33] phosphorus,[34] arsenic,[35] selenium,[36] antimony,[37] tellurium[38] and iodine.[39]
Nonmetallic elements are either shiny, colored, or colorless. The shiny appearance of boron, graphitic carbon, silicon, black phosphorus, germanium, arsenic, selenium, antimony, tellurium, and iodine is a result of their structures featuring varying degrees of delocalised (free-moving) electrons that scatter incoming visible light.[40] The colored nonmetals (sulfur, fluorine, chlorine, bromine) absorb some colors (wavelengths) and transmit the complementary or opposite colors. In the case of chlorine, for example, Elliot writes that its "familiar yellow-green colour...is due to a broad region of absorption in the violet and blue regions of the spectrum".[41][n 10]
For the colorless nonmetals (hydrogen, nitrogen, oxygen, and the noble gases), their electrons are held sufficiently strongly such that no absorption happens in the visible part of the spectrum, and all visible light is transmitted.[43]
The electrical and thermal conductivities of nonmetals and the brittle nature of the solids are likewise related to their internal arrangements. Whereas good conductivity and plasticity (malleability, ductility) are ordinarily associated with the presence of free-moving and uniformly distributed electrons in metals[44] the electrons in nonmetals typically lack such mobility.[45] Among the nonmetallic elements, good electrical and thermal conductivity is seen only in carbon, arsenic, and antimony.[n 11] Good thermal conductivity otherwise occurs only in boron, silicon, phosphorus, and germanium;[23] such conductivity is transmitted though vibrations of the crystalline lattices of these elements.[46] Moderate electrical conductivity is evidenced in boron, silicon, phosphorus, germanium, selenium, tellurium, and iodine.[n 12] Plasticity occurs under limited circumstances in carbon, as exfoliated (expanded) graphite[48] and as carbon nanotube wire;[49] phosphorus as white phosphorus (soft as wax, pliable and can be cut with a knife, at room temperature);[50] sulfur as plastic sulfur;[51] and selenium as selenium wires, drawn from the molten form.[52]
Chemical
| Aspect | Metals | Nonmetals |
|---|---|---|
| Electronegativity | Lower than nonmetals, with some exceptions[54] |
Relatively high |
| Chemical bonding | ||
| Seldom form covalent bonds |
Frequently form covalent bonds | |
| Metallic bonds (alloys) between metals |
Covalent bonds between nonmetals | |
| Ionic bonds between nonmetals and metals | ||
| Oxidation states |
Positive | Negative or positive |
| Oxides | Basic in lower oxides; increasingly acidic in higher oxides |
Acidic; never basic[55] |
| In aqueous solution[56] |
Exist as cations | Exist as anions or oxyanions |
Nonmetals have relatively high values of electronegativity[7] and tend to form acidic compounds. For example, the solid nonmetals (including metalloids) react with nitric acid to form either an acid, or an oxide that has acidic properties predominating.[n 13]
They tend to gain or share electrons when they react, unlike metals which tend to donate electrons. Given the stability of the electron configurations of the noble gases (which have full outer shells), nonmetals generally gain enough electrons to give them the electron configuration of the following noble gas, whereas metals tend to lose electrons sufficient to leave them with the electron configuration of the preceding noble gas. For nonmetallic elements this tendency is summarized in the duet and octet rules of thumb (and for metals there is a less rigorously predictive 18-electron rule).[59]
Nonmetals further mostly have higher ionization energies, electron affinities, and standard reduction potentials than metals. In general, the higher these values are (including electronegativity) the more nonmetallic the element is.[60]
The chemical differences between metals and nonmetals largely arise from the attractive force between the positive nuclear charge of an individual atom and its negatively charged outer electrons. From left to right across each period of the periodic table the nuclear charge increases as the number of protons in the atomic nucleus increases.[61] There is an associated reduction in atomic radius[62] as the increasing nuclear charge draws the outer electrons closer to the core.[63] In metals, the effect of the nuclear charge is generally weaker than for nonmetallic elements. In bonding, metals therefore tend to lose electrons, and form positively charged or polarized atoms or ions whereas nonmetals tend to gain those same electrons due to their stronger nuclear charge, and form negatively charged ions or polarized atoms.[64]
The number of compounds formed by nonmetals is vast.[65] The first 10 places in a "top 20" table of elements most frequently encountered in 895,501,834 compounds, as listed in the Chemical Abstracts Service register for November 2, 2021, were occupied by nonmetals. Hydrogen, carbon, oxygen, and nitrogen were collectively found in the majority (80%) of compounds. Silicon, a metalloid, was in 11th place. The highest rated metal, with an occurrence frequency of 0.14%, was iron, in 12th place.[66] A few examples of nonmetal compounds are: boric acid (H3BO3), used in ceramic glazes;[67] selenocysteine (C3H7NO2Se), the 21st amino acid of life;[68] phosphorus sesquisulfide (P4S3), in strike anywhere matches;[69] and teflon ((C2F4)n),[70] as used in non-stick coatings for pans and other cookware.
Complications
Complicating the chemistry of the nonmetals are the anomalies seen in the first row of each periodic table block. These anomalies are prominent in hydrogen, boron (whether as a nonmetal or metalloid), carbon, nitrogen, oxygen, and fluorine. In later rows they manifest as secondary periodicity or non-uniform periodic trends going down most of the p-block groups,[71] and unusual oxidation states in the heavier nonmetals.
First row anomaly
Starting with hydrogen, the first row anomaly largely arises from the electron configurations of the elements concerned. Hydrogen is noted for the different ways it forms bonds. It most commonly forms covalent bonds. It can lose its single electron in aqueous solution, leaving behind a bare proton with tremendous polarizing power.[72] This consequently attaches itself to the lone electron pair of an oxygen atom in a water molecule, thereby forming the basis of acid-base chemistry.[73] A hydrogen atom in a molecule can form a second, weaker, bond with an atom or group of atoms in another molecule. According to Cressey such bonding, "helps give snowflakes their hexagonal symmetry, binds DNA into a double helix; shapes the three-dimensional forms of proteins; and even raises water's boiling point high enough to make a decent cup of tea."[74]
Hydrogen and helium, and boron to neon, have unusually small atomic radii. This occurs because the 1s and 2p subshells have no inner analogues (that is, there is no zero shell and no 1p subshell), and they therefore experience no electron repulsion effects, unlike the 3p, 4p, and 5p subshells of heavier elements.[75] Ionization energies and electronegativities among these elements are consequently higher than would otherwise be expected, having regard to periodic trends. The small atomic radii of carbon, nitrogen, and oxygen facilitate the formation of double or triple bonds.[76]
While it would normally be expected that hydrogen and helium, on electron configuration consistency grounds, would be located atop the s-block elements, the first row anomaly in these two elements is strong enough to warrant alternative placements. Hydrogen is occasionally positioned over fluorine, in group 17, rather than over lithium in group 1. Helium is regularly positioned over neon, in group 18, rather than over beryllium in group 2.[77]
Secondary periodicity
Immediately after the first row of d-block metals, scandium to zinc, the 3d electrons in the p-block elements—that is, gallium (a metal), germanium, arsenic, selenium, and bromine—are not as effective at shielding the increased positive nuclear charge. A similar effect accompanies the appearance of fourteen f-block metals between barium and lutetium, ultimately resulting in smaller than expected atomic radii for the elements from hafnium (Hf) onwards.[78] The net result, especially for the group 13–15 elements, is that there is an alternation in some periodic trends going down groups 13 to 17.[79]
Unusual oxidation states
The larger atomic radii of the heavier group 15–18 nonmetals enable higher bulk coordination numbers, and result in lower electronegativity values that better tolerate higher positive charges. The elements involved are thereby able to exhibit oxidation states other than the lowest for their group (that is, 3, 2, 1, or 0), for example in phosphorus pentachloride (PCl5), sulfur hexafluoride (SF6), iodine heptafluoride (IF7), and xenon difluoride (XeF2).[80]
Subclasses
Approaches to classifying nonmetals may involve from as few as two subclasses to up to six or seven. For example, the Encyclopædia Britannica periodic table recognizes noble gases, halogens, and other nonmetals, and splits the elements commonly recognized as metalloids between "other metals" and "other nonmetals".[92] The Royal Society of Chemistry periodic table instead uses a different color for each of its eight main groups, and nonmetals can be found in seven of these.[93]
From right to left in periodic table terms, three or four kinds of nonmetals are more or less commonly discerned. These are:
- the relatively inert noble gases;[94]
- a set of chemically strong halogen elements—fluorine, chlorine, bromine and iodine—sometimes referred to as nonmetal halogens[95] or halogen nonmetals[96] (the term used here) or stable halogens;[97]
- a set of unclassified nonmetals, including elements such as hydrogen, carbon, nitrogen, and oxygen, with no widely recognized collective name;[n 18] and
- the chemically weak nonmetallic metalloids[106] sometimes considered to be nonmetals and sometimes not.[n 19]
Since the metalloids occupy "frontier territory",[108] where metals meet nonmetals, their treatment varies from author to author. Some consider them separate from both metals and nonmetals; some regard them as nonmetals[109] or as a sub-class of nonmetals.[110] Other authors count some of them as metals, for example arsenic and antimony, due to their similarities to heavy metals.[111][n 20] Metalloids are here treated as nonmetals in light of their chemical behavior,[106] and for comparative purposes.
Aside from the metalloids, some boundary fuzziness and overlapping (as occurs with classification schemes generally),[112] can be discerned among the other nonmetal subclasses. Carbon, phosphorus, selenium, and iodine border the metalloids and show some metallic character, as does hydrogen. Among the noble gases, radon is the most metallic and begins to show some cationic behavior, which is unusual for a nonmetal.[113]
Noble gases
Six nonmetals are classified as noble gases: helium, neon, argon, krypton, xenon, and the radioactive radon. In conventional periodic tables they occupy the rightmost column. They are called noble gases in light of their characteristically very low chemical reactivity.[94]
They have very similar properties, with all of them being colorless, odorless, and nonflammable. With their closed outer electron shells the noble gases have feeble interatomic forces of attraction, resulting in very low melting and boiling points.[114] That is why they are all gases under standard conditions, even those with atomic masses larger than many normally solid elements.[115]
Chemically, the noble gases have relatively high ionization energies, nil or negative electron affinities, and high to very high electronegativities. Compounds of the noble gases number in the hundreds, and the list continues to grow,[116] with most of these involving oxygen or fluorine combining with either krypton, xenon or radon.[117]
In periodic table terms, an analogy can be drawn between the noble gases and noble metals (such as platinum and gold) which are similarly reluctant to combine with other elements.[118] As a further example, xenon, in the +8 oxidation state, forms a pale yellow explosive oxide, XeO4, while osmium, another noble metal, forms a yellow, strongly oxidizing oxide,[119] OsO4. There are parallels, too, in the formulas of the oxyfluorides: XeO2F4 and OsO2F4, and XeO3F2 and OsO3F2.[120]
About 1015 tonnes of noble gases are present in the Earth's atmosphere.[121] Additionally, natural gas is found to be as much as 7% Helium.[122] Radon diffuses out of rocks, where it is formed during the natural decay sequence of uranium and thorium.[123] The Earth's core may contain about 1013 tons of xenon, in the form of stable XeFe3 and XeNi3 intermetallic compounds. This may explain why "studies of the Earth's atmosphere have shown that more than 90% of the expected amount of Xe is depleted."[124]
Halogen nonmetals

While the halogen nonmetals are markedly reactive and corrosive elements, they can be found in such mundane compounds as toothpaste (NaF); ordinary table salt (NaCl); swimming pool disinfectant (NaBr); or food supplements (KI). The word "halogen" means "salt former".[126]
Physically, fluorine and chlorine are pale yellow and yellowish green gases; bromine is a reddish-brown liquid (usually topped by a layer of its fumes); and iodine, under white light, is a metallic-looking[81] solid. Electrically, the first three are insulators while iodine is a semiconductor (along its planes).[127]
Chemically, they have high ionization energies, electron affinities, and electronegativity values, and are mostly relatively strong oxidizing agents.[128] Manifestations of this status include their corrosive nature.[129] All four exhibit a tendency to form predominately ionic compounds with metals[130] whereas the remaining nonmetals, bar oxygen, tend to form predominately covalent compounds with metals.[n 21] The reactive and strongly electronegative nature of the halogen nonmetals represents the epitome of nonmetallic character.[134]
In periodic table terms, the counterparts of the highly nonmetallic halogens in group 17 are the highly reactive alkali metals, such as sodium and potassium, in group 1.[135] Most of the alkali metals, as if in imitation of the halogen nonmetals, are known to form –1 anions (something that rarely occurs among metals).[136]
The halogen nonmetals are found in salt-related minerals. Fluorine occurs in fluorite (CaF2), a widespread mineral. Chlorine, bromine, and iodine are found in brines. Exceptionally, a 2012 study reported the presence of 0.04% native fluorine (F2) by weight in antozonite, attributing these inclusions as a result of radiation from the presence of tiny amounts of uranium.[137]
Metalloids
The six elements more commonly recognized as metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium, each having a metallic appearance. On a standard periodic table, they occupy a diagonal area in the p-block extending from boron, at the upper left, to tellurium, at lower right, along the dividing line between metals and nonmetals shown on some tables.[2]
They are brittle and poor-to-good conductors of heat and electricity. Boron, silicon, germanium, and tellurium are semiconductors. Arsenic and antimony have the electronic structures of semimetals, although both have less stable semiconducting forms.[2]
Chemically, the metalloids generally behave like (weak) nonmetals. Among the nonmetallic elements they tend to have the lowest ionization energies, electron affinities, and electronegativity values, and are relatively weak oxidizing agents. They further demonstrate a tendency to form alloys with metals.[2]
In periodic-table terms, to the left of the weakly nonmetallic metalloids are an indeterminate set of weakly metallic metals (such as tin, lead and bismuth)[139] sometimes referred to as post-transition metals.[140] Dingle explains the situation this way:
... with 'no-doubt' metals on the far left of the table, and no-doubt non-metals on the far right ... the gap between the two extremes is bridged first by the poor (post-transition) metals, and then by the metalloids—which, perhaps by the same token, might collectively be renamed the 'poor non-metals'.[141]
The metalloids tend to be found in forms combined with oxygen, sulfur, or (in the case of tellurium) gold or silver.[142] Boron is found in boron-oxygen borate minerals, including in volcanic spring waters. Silicon occurs in the silicon-oxygen mineral silica (sand). Germanium, arsenic, and antimony are mainly found as components of sulfide ores. Tellurium occurs in telluride minerals of gold or silver. Native forms of arsenic, antimony, and tellurium have been reported.[143]
Unclassified nonmetals
After the nonmetallic elements are classified as either noble gases, halogens, or metalloids, the remaining seven nonmetals are hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, and selenium. In their most stable forms, three are colorless gases (H, N, O); three have a metal-like appearance (C, P, Se); and one is yellow (S). Electrically, graphitic carbon is a semimetal along its planes[145] and a semiconductor in a direction perpendicular to its planes;[146] phosphorus and selenium are semiconductors;[147] and hydrogen, nitrogen, oxygen, and sulfur are insulators.[n 22]
These elements are generally regarded as being too diverse to merit a collective classification,[149] and have been referred to as other nonmetals,[150] or more plainly as nonmetals, located between the metalloids and the halogens.[151] Consequently, their chemistry tends to be taught disparately, according to their four respective periodic table groups.[152] For example: hydrogen in group 1; the group 14 nonmetals (carbon, and possibly silicon and germanium); the group 15 nonmetals (nitrogen, phosphorus, and possibly arsenic and antimony); and the group 16 nonmetals (oxygen, sulfur, selenium, and possibly tellurium). Other subdivisions are possible according to the individual preferences of authors.[n 23]
Hydrogen, in particular, behaves in some respects like a metal and in others like a nonmetal.[154] Like a metal, it can (first) lose its single electron;[155] it can stand in for alkali metals in typical alkali metal structures;[156] and is capable of forming alloy-like hydrides, featuring metallic bonding, with some transition metals.[157] On the other hand, it is an insulating diatomic gas, like a typical nonmetal, and in chemical reactions has a tendency to eventually attain the electron configuration of helium.[158] It does this by way of forming a covalent or ionic bond[157] or, if it has lost its electron, attaching itself to a lone pair of electrons.[159]
Some or all of these nonmetals nevertheless have several shared properties. Most of them, being less reactive than the halogens,[160] can occur naturally in the environment.[161] They have prominent biological[162] and geochemical roles.[149] While their physical and chemical character is "moderately non-metallic", on a net basis,[149] all of them have corrosive aspects. Hydrogen can corrode metals. Carbon corrosion can occur in fuel cells.[163] Acid rain is caused by dissolved nitrogen or sulfur. Oxygen corrodes iron via rust. White phosphorus, the most unstable form, ignites in air and produces phosphoric acid residue.[164] Untreated selenium in soils can give rise to corrosive hydrogen selenide gas.[165] When combined with metals, the unclassified nonmetals can form high hardness (interstitial or refractory) compounds,[166] on account of their relatively small atomic radii and sufficiently low ionization energies.[149] They show a tendency to bond to themselves, especially in solid compounds.[167] Diagonal periodic table relationships among these nonmetals echo similar relationships among the metalloids.[168]
In periodic-table terms, a geographic analogy is seen between the unclassified nonmetals and transition metals. The unclassified nonmetals occupy territory between the strongly nonmetallic halogens, on the right, and the weakly nonmetallic metalloids, on the left. The transition metals occupy territory, between the "virulent and violent" metals on the left of the periodic table, and the "calm and contented” metals to the right and form a "transitional bridge" between the two.[169]
Unclassified nonmetals typically occur in elemental forms (oxygen, sulfur) or are found in association with either of these two elements:[142]
- Hydrogen occurs in the world's oceans as a component of water, and in natural gas as a component of methane and hydrogen sulfide.[170]
- Carbon occurs in limestone, dolomite, and marble, as carbonates.[171] Less well known is carbon as graphite, which mainly occurs in metamorphic silicate rocks,[172] as a result of the compression and heating of sedimentary carbon compounds.[173]
- Oxygen is found in the atmosphere; in the oceans as a component of water; and in the crust as oxide minerals.[174]
- Phosphorus minerals are widespread, usually as phosphorus-oxygen phosphates.[175]
- Elemental sulfur can be found in or near hot springs and volcanic regions in many parts of the world; sulfur minerals are widespread, usually as sulfides or oxygen-sulfur sulfates.[176]
- Selenium occurs in metal sulfide ores, where it partially replaces the sulfur; elemental selenium is occasionally found.[177]
Allotropes
Most nonmetallic elements exist in allotropic forms. Carbon, for example, occurs as graphite, diamond, and other forms. Such allotropes may exhibit physical properties that are more metallic or less nonmetallic.[178]
Among the halogen nonmetals, and unclassified nonmetals:
- Iodine is known in a semiconducting amorphous form.[179]
- Graphite, the standard state of carbon, is a fairly good electrical conductor. The diamond allotrope of carbon is clearly nonmetallic, being translucent and an extremely poor electrical conductor.[180] Carbon is known in several other allotropic forms, including semiconducting buckminsterfullerene,[181] and amorphous[182] and paracrystalline (mixed amorphous and crystalline)[183] varieties.
- Nitrogen can form gaseous tetranitrogen (N4), an unstable polyatomic molecule with a lifetime of about one microsecond.[184]
- Oxygen is a diatomic molecule in its standard state; it also exists as ozone (O3), an unstable nonmetallic allotrope with an "indoors" half-life of around half an hour, compared to about three days in ambient air at 20 °C.[185]
- Phosphorus, uniquely, exists in several allotropic forms that are more stable than its standard state as white phosphorus (P4). The white, red, and black allotropes are probably the best known; the first is an insulator; the latter two are semiconductors.[186] Phosphorus also exists as diphosphorus (P2), an unstable diatomic allotrope.[187]
- Sulfur has more allotropes than any other element.[188] Amorphous sulfur, a metastable mixture of such allotropes, is noted for its elasticity.[189]
- Selenium has several nonmetallic allotropes, all of which are much less electrically conducting than its standard state of gray "metallic" selenium.[190]
All the elements most commonly recognized as metalloids form allotropes:
- Boron is known in several crystalline and amorphous forms.[191]
- Silicon can form crystalline (diamond-like); amorphous; and orthorhombic Si24 allotropes.[192]
- At a pressure of about 10–11 GPa, germanium transforms to a metallic phase with the same tetragonal structure as tin. When decompressed—and depending on the speed of pressure release—metallic germanium forms a series of allotropes that are metastable in ambient conditions.[193]
- Arsenic and antimony form several well-known allotropes (yellow, grey, and black).[194]
- Tellurium is known in crystalline and amorphous forms.[195]
Other allotropic forms of nonmetallic elements are known, either under pressure or in monolayers. Under sufficiently high pressures, at least half of the nonmetallic elements that are semiconductors or insulators,[n 24] starting with phosphorus at 1.7 GPa, have been observed to form metallic allotropes.[197][n 25] Single layer two-dimensional forms of nonmetals include borophene (boron), graphene (carbon), silicene (silicon), phosphorene (phosphorus), germanene (germanium), arsenene (arsenic), antimonene (antimony), and tellurene (tellurium), collectively referred to as xenes.[199]
Prevalence and access
Abundance
| Domain | Main components | Next most abundant |
|---|---|---|
| Crust | O 61%, Si 20% | H 2.9% |
| Atmosphere | N 78%, O 21% | Ar 0.5% |
| Hydrosphere | O 66.2%, H 33.2% | Cl 0.3% |
| Biomass | O 63%, C 20%, H 10% | N 3.0% |
Hydrogen and helium are estimated to make up approximately 99% of all ordinary matter in the universe and over 99.9% of its atoms.[201] Oxygen is thought to be the next most abundant element, at about 0.1%.[202] Less than five per cent of the universe is believed to be made of ordinary matter, represented by stars, planets, and living beings. The balance is hypothesized to be made of dark energy and dark matter, both of which are currently poorly understood.[203]
Five nonmetals—namely hydrogen, carbon, nitrogen, oxygen and silicon—constitute the bulk of the Earth's crust, atmosphere, hydrosphere, and biomass, in the quantities shown in the table.
Extraction
Nonmetals, and metalloids, are extracted in their raw forms from:[161]
- brine—chlorine, bromine, iodine;
- liquid air—nitrogen, oxygen, neon, argon, krypton, xenon;
- minerals—boron (borate minerals); carbon (coal; diamond; graphite); fluorine (fluorite); silicon (silica); phosphorus (phosphates); antimony (stibnite, tetrahedrite); iodine (in sodium iodate and sodium iodide);
- natural gas—hydrogen, helium, sulfur; and
- ores, as processing byproducts—germanium (zinc ores); arsenic (copper and lead ores); selenium, tellurium (copper ores); and radon (uranium-bearing ores).
Cost
Day to day costs will vary depending on purity, quantity,[n 26] market conditions, and supplier surcharges.[208]
Based on the available literature as of April 2023, the cited costs of most nonmetals are less than the $US0.74 per gram cost of silver.[209] The exceptions are boron, phosphorus, germanium, xenon, and radon (notionally):
- Boron costs around $25 per gram for 99.7% pure polycrystalline chunks with a particle size of about 1 cm.[210] Earlier, in 1997, boron was quoted at $280 per gram for polycrystalline 4-to-6-mm-diameter rods of 99.999% purity,[211] about 10 times the then $28.35 per gram cost of gold.[212]
- In 2020, phosphorus in its most-stable black form could "cost up to $1,000 per gram",[213] more than 15 times the cost of gold, whereas ordinary red phosphorus, in 2017, was priced at about $3.40 per kilogram.[214] Researchers hoped to be able to reduce the cost of black phosphorus to as low as $1 per gram.[213]
- Germanium and xenon cost about $1.30 and $7.60 per gram.[215]
- Up to 2013, radon was available from the National Institute of Standards and Technology for $1,636 per 0.2 ml unit of issue, equivalent to about $86,000,000 per gram, with no indication of a discount for bulk quantities.[216]
Uses
The distinctive properties of nonmetallic elements allow for specific applications that often cannot be fulfilled by metallic elements alone. Living organisms are composed almost entirely of the nonmetals hydrogen, oxygen, carbon, and nitrogen. Nonmetallic elements are important to industries ranging from electronics and energy storage to agriculture and chemical production, for example:
- Carbon fibers possess high strength and low weight, making them ideal for applications in aerospace, sports equipment, and Automotive industry . Graphene, a single layer of carbon atoms, has exceptional electrical and thermal conductivity, making it valuable for electronic devices, energy storage, and composite materialss.[217]
- Nitrogen is a key component in the production of fertilizers, which enhance crop growth and agricultural productivity. It's low temperature properties make it useful for cryogenic applications, such as preserving biological samples and freezing food.[218]
- In life support, oxygen is vital for human respiration, and it is used in medical settings to assist patients with respiratory difficulties. It supports combustion and is used in various industrial processes, such as metal smelting and waste incineration.[219]
- In semiconductors, silicon is the backbone of the electronics industry. It is used to manufacture computer chips, solar cells, and various electronic components. Silicon dioxide (silica) is used in the production of glass, ceramics, and optical fibers, enabling applications in windows, lenses, and communication networks.[220]
- Sulfur is used in the production of sulfuric acid, one of the most widely used industrial chemicals. It is also used in the synthesis of various organic compounds. Sulfur compounds are crucial in the vulcanization process, which imparts strength and elasticity to rubber products.[221]
Shared uses of different subsets of the nonmetals encompass their presence in, or specific uses in the fields of air replacements (inert); dyestuffs; flame retardants or extinguishers; household accoutrements; lasers and lighting; mineral acids; plug-in hybrid vehicles; and welding gases.[161][222] To the extent that metalloids show metallic character, they have speciality uses extending to (for example) oxide glasses and alloying components.[223]
History, background, and taxonomy
Discovery
Most nonmetals were discovered in the 18th and 19th centuries. Before then, carbon, sulfur, and antimony were known in antiquity; arsenic was discovered during the Middle Ages (by Albertus Magnus); and Hennig Brand isolated phosphorus from urine in 1669. Helium (1868) holds the distinction of being the only element not first discovered on Earth.[n 27] Radon is the most recently discovered nonmetal, being found only at the end of the 19th century.[161]
Chemistry- or physics-based techniques used in the isolation efforts were spectroscopy, fractional distillation, radiation detection, electrolysis, ore acidification, displacement reactions, combustion, and heating; a few nonmetals occurred naturally as free elements:
- Of the noble gases, helium was detected via its yellow line in the coronal spectrum of the sun, and later by observing the bubbles escaping from uranite UO2 dissolved in acid. Neon through xenon were obtained via fractional distillation of air. Radon was first observed emanating from compounds of thorium, three years after Henri Becquerel's discovery of radiation in 1896.[225]
- The halogen nonmetals were obtained from their halides via either electrolysis, adding an acid, or displacement. Some chemists died as a result of their experiments trying to isolate fluorine.[226]
- Among the unclassified nonmetals, carbon was known (or produced) as charcoal, soot, graphite, and diamond; nitrogen was observed in air from which oxygen had been removed; oxygen was obtained by heating mercurous oxide; phosphorus was liberated by heating ammonium sodium hydrogen phosphate (Na(NH4)HPO4), as found in urine;[227] sulfur occurred naturally as a free element; and selenium[n 28] was detected as a residue in sulfuric acid.[229]
- Most of the elements commonly recognized as metalloids were isolated by heating their oxides (boron, silicon, arsenic, tellurium) or a sulfide (germanium).[161] Antimony was known in its native form, as well as being attainable by heating its sulfide.[230]
Origin of the concept
The distinction between metals and nonmetals arose, in a convoluted manner, from a crude recognition of different kinds of matter, namely pure substances, mixtures, compounds and elements. Thus, matter could be divided into pure substances (such as salt, bicarb of soda, or sulfur) and mixtures (aqua regia, gunpowder, or bronze, for example); and pure substances eventually could be distinguished as compounds and elements.[231] "Metallic" elements then seemed to have broadly distinguishable attributes that other elements did not, such as their ability to conduct heat or for their "earths" (oxides) to form basic solutions in water, for example as occurred with quicklime (CaO).[232]
Use of the term
The term nonmetallic dates from as far back as 1566. In a medical treatise published that year, Loys de L’Aunay (a French doctor) described the different properties of plant substances from metallic and "non-metallic" land.[233]
In early chemistry, Wilhelm Homberg (a German natural philosopher) referred to "non-metallic" sulfur in Des Essais de Chimie (1708).[234] He questioned the five-fold division of all matter into sulfur, mercury, salt, water, and earth, as postulated by Étienne de Clave (1641) in the New Philosophical Light of True Principles and Elements of Nature.[235] Homberg's approach represented "an important move toward the modern concept of an element".[236]
Lavoisier, in his "revolutionary"[237] 1789 work Traité élémentaire de chimie, published the first modern list of chemical elements, in which he distinguished between gases, metals, nonmetals, and earths (heat resistant oxides).[238] In its first seventeen years, Lavoisier's work was republished in twenty-three editions in six languages, and "carried ... [his] new chemistry all over Europe and America."[239]
Suggested distinguishing criteria
Physical
|
|
Chemical
Electron related
|
In 1809, Humphry Davy's discovery of sodium and potassium "annihilated"[262] the line of demarcation between metals and nonmetals. Before then, metals had been distinguished on the basis of their ponderousness or relatively high densities.[263] Sodium and potassium, on the other hand, floated on water and yet were clearly metals on the basis of their chemical behaviour.[264]
From as early as 1811, different properties—physical, chemical, and electron related—have been used in attempts to refine the distinction between metals and nonmetals. The accompanying table sets out 22 such properties, ordered by type and date of discovery.
Probably the most well-known property is that the electrical conductivity of a metal increases when temperature falls, whereas that of a nonmetal rises.[252] However, this does not follow for plutonium, carbon, arsenic, and antimony. The electrical conductivity of plutonium is increased when this metal is heated within a temperature range of –175 to +125 °C.[265] The electrical conductivity of carbon, despite being widely regarded as a nonmetal, is likewise increased when heated.[266] Arsenic and antimony are sometimes classified as nonmetals, yet act similarly to carbon.[267]
Kneen et al. suggested that the nonmetals could be distinguished once a [single] criterion for metallicity had been chosen, adding that, "many arbitrary classifications are possible, most of which, if chosen reasonably, would be similar but not necessarily identical."[10] Emsley noted that, "No single property ... can be used to classify all the elements as either metals or nonmetals."[268] Jones added that "classes are usually defined by more than two attributes".[269]
| EN | ||
| Density | < 1.9 | ≥ 1.9 |
| < 7 gm/cm3 | Groups 1 and 2 Sc, Y, La Ce, Pr, Eu, Yb Ti, Zr, V; Al, Ga |
Noble gases F, Cl, Br, I H, C, N, P, O, S, Se B, Si, Ge, As, Sb, Te^ |
| > 7 gm/cm3 | Nd, Pm, Sm, Gd, Tb, Dy Ho, Er, Tm, Lu; Ac–Es; Hf, Nb, Ta; Cr, Mn, Fe, Co, Zn, Cd, In, Tl, Pb |
Ni, Mo, W, Tc, Re, Platinum group metals, Coinage metals, Hg; Sn, Bi, Po, At |
| ^ italicized elements are commonly recognised by some authors as metalloids | ||
Johnson suggested that physical properties can best indicate the metallic or nonmetallic properties of an element, with the proviso that other properties will be needed in ambiguous cases. He observed that all gaseous or nonconducting elements are nonmetals; solid nonmetals are hard and brittle or soft and crumbly, whereas metals are usually malleable and ductile; and nonmetal oxides are acidic.[274]
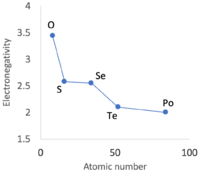
According to Hein and Arena, nonmetals have relatively low densities and high electronegativity;[7] the accompanying table bears this out. Nonmetallic elements occupy the top left quadrant, where densities are relatively low and electronegativity values relatively high. The other three quadrants are occupied by metals. Some authors further divide the elements into metals, metalloids, and nonmetals, although Odberg argues that anything not a metal is, by rules of categorisation, a nonmetal.[275]
Development of subclasses
A basic taxonomy of nonmetals was set out in 1844, by Alphonse Dupasquier, a French doctor, pharmacist and chemist.[276] To facilitate the study of nonmetals, he wrote:[277]
- They will be divided into four groups or sections, as in the following:
- Organogens O, N, H, C
- Sulphuroids S, Se, P
- Chloroides F, Cl, Br, I
- Boroids B, Si.
An echo of Dupasquier's fourfold classification is seen in the modern subclasses. The organogens and sulphuroids represent the set of unclassified nonmetals. The chloroide nonmetals came to be independently referred to as halogens.[278] The boroid nonmetals were expanded into the metalloids, starting from as early as 1864.[279] The noble gases, as a discrete grouping, were counted among the nonmetals from as early as 1900.[280]
Comparison
Some properties of metals, metalloids, unclassified nonmetals, halogen nonmetals, and noble gases are summarized in the following table.[n 31] Physical properties apply to elements in their most stable forms under ambient conditions, and are listed in loose order of ease of determination. Chemical properties are listed from general to descriptive, and then to specific. The dashed line around the metalloids denotes that, depending on the author, the elements involved may or may not be recognized as a distinct class or subclass of elements. Metals are included as a reference point.
Most properties show a left-to-right progression in metallic-to-nonmetallic character or average values. The periodic table can thus be indicatively divided into metals and nonmetals, with more or less distinct gradations seen among the nonmetals.[281]
| Physical property | Metals alkali, alkaline earth, lanthanide, actinide, transition, post-transition |
Metalloids boron, silicon, germanium, arsenic, antimony, tellurium |
Unclassified nonmetals hydrogen, carbon, nitrogen, phosphorus, oxygen, sulfur, selenium |
Halogen nonmetals fluorine, chlorine, bromine, iodine |
Noble gases helium, neon, argon, krypton, xenon, radon |
|---|---|---|---|---|---|
| Form and heft[282] |
|
|
|
|
|
| Appearance | lustrous[21] | lustrous[285] | colorless[290] | ||
| Elasticity | mostly malleable and ductile[21] (Hg is liquid) | brittle[285] | C, black P, S, Se brittle; all four have less stable non-brittle forms[291][n 32] | iodine is brittle[293] | not applicable |
| Electrical conductivity | good[n 33] |
|
|
|
poor[n 37] |
| Electronic structure[196] | metallic (Bi is a semimetal) | semimetal (As, Sb) or semiconductor |
|
semiconductor (I) or insulator | insulator |
| Chemical property | Metals alkali, alkaline earth, lanthanide, actinide, transition, post-transition |
Metalloids boron, silicon, germanium, arsenic, antimony, tellurium |
Unclassified nonmetals hydrogen, carbon, nitrogen, phosphorus, oxygen, sulfur, selenium |
Halogen nonmetals fluorine, chlorine, bromine, iodine |
Noble gases helium, neon, argon, krypton, xenon, radon |
| General chemical behavior |
|
weakly nonmetallic[n 38] | moderately nonmetallic[299] | strongly nonmetallic[300] | |
| Oxides | |||||
| Compounds with metals | alloys[21] or intermetallic compounds[318] | tend to form alloys or intermetallic compounds[319] | mainly ionic[130] | simple compounds in ambient conditions not known[n 41] | |
| Ionization energy (kJ mol−1)‡ [323] |
|
|
|
|
|
| Electronegativity (Pauling)[n 42]‡ [325] |
|
|
|
|
|
| † Hydrogen can also form alloy-like hydrides[326] ‡ The labels low, moderate, high, and very high are arbitrarily based on the value spans listed in the table | |||||
See also
- CHON (carbon, hydrogen, oxygen, nitrogen)
- List of nonmetal monographs
- Metallization pressure
- Nonmetal (astrophysics)
- Nonmetal (physics)
- Period 1 elements (hydrogen, helium)
- Properties of nonmetals (and metalloids) by group
Notes
- ↑ H; N; O, S; F, Cl, Br, I; He, Ne, Ar, Kr, Xe, Rn.[1]
- ↑ C; P; Se.[1] On the other hand, these three elements were counted as metalloids in a survey of 194 lists of metalloids, 16, 10, and 46 times respectively.[2]
- ↑ B; Si, Ge; As, Sb; Te.[3]
- ↑ Al, Ga, In, Tl; Sn, Pb; Bi; Po; At.
- ↑ Hydrogen has historically been placed over one or more of lithium, boron,[4] carbon, or fluorine;[5] or over no group at all; or over all main groups simultaneously, and therefore may or may not be adjacent to other nonmetals.[6]
- ↑ Metallic or nonmetallic character is usually taken to be indicated by one property rather than two or more properties.
- ↑ Steudel's monograph is an updated translation of the fifth German edition of 2013, incorporating the literature up to Spring 2019.
- ↑ Solid iodine has a silvery metallic appearance under white light at room temperature.[19] It volatizes at ordinary and higher temperatures, passing from solid to gas; its vapours are violet-colored.[20]
- ↑ The solid nonmetals have thermal conductivity values of from 0.27 W m–1 K–1 for sulfur to 2,000 for carbon cf. 6.3 for neptunium to 429 for silver, both metals;[23] electrical conductivity values range from 10−18 S•cm−1 for sulfur[23] to 3 × 104 in graphite[24] or 3.9 × 104 for arsenic[25] cf. 0.69 × 104 for manganese to 63 × 104 for silver, metals both.[23]
- ↑ The absorbed light may be converted to heat or re-emitted in all directions so that the emission spectrum is thousands of times weaker than the incident light radiation.[42]
- ↑ Thermal conductivity values for metals range from 6.3 W m–1 K–1 for neptunium to 429 for silver; cf. antimony 24.3, arsenic 50, and carbon 2000;[23] electrical conductivity values of metals range from 0.69 S•cm−1 × 104 for manganese to 63 × 104 for silver; cf. carbon 3 × 104,[24] arsenic 3.9 × 104 and antimony 2.3 × 104.[23]
- ↑ These elements being semiconductors.[47]
- ↑ Acids are formed by boron, phosphorus, selenium, arsenic, iodine;[57] oxides by carbon, silicon, germanium, sulfur, antimony, and tellurium.[58]
- ↑ These elements are hydrogen and helium in the s-block; boron to neon in the p-block; scandium to zinc in the d-block; and lanthanum to ytterbium in the f-block.
- ↑ Noble gases: He, Ne, Ar, Kr, Xe, Rn; Halogen nonmetals: F, Cl, Br, I; Unclassified nonmetals: H, C, N, P, O, S, Se; Metalloids: B, Si, Ge, As, Sb, Te. Nearby metals are Al, Ga, In, Tl; Sn, Pb; Bi; Po; and At.
- ↑ The seven nonmetals marked with single or double daggers each have a lackluster appearance and discrete molecular structures, but for I which has a metallic appearance under white light.[81] The remaining reactive nonmetallic elements have giant covalent structures, but for H which is a diatomic gas.[82]
The single dagger nonmetals N, S and iodine are somewhat hobbled as "strong" nonmetals.
While N has a high electronegativity, it is a reluctant anion former,[83] and a pedestrian oxidizing agent unless combined with a more active nonmetal like O or F.[84]
S reacts in the cold with alkalic and post-transition metals, and Cu, Ag and Hg,[85] but otherwise has low values of ionization energy, electron affinity, and electronegativity compared to the averages of the others; it is regarded as being not a particularly good oxidizing agent.[86]
Iodine is sufficiently corrosive to cause lesions resembling thermal burns, if handled without suitable protection,[87] and tincture of iodine will smoothly dissolve Au.[88] That said, while "F, Cl and Br will all oxidize Fe2+ (aq) to Fe3+(aq) ... iodine ... is such a [relatively] weak oxidizing agent that it cannot remove electrons from Fe(II) ions to form Fe(III) ions."[89] Thus, for the reaction X2 + 2e− → 2X−(aq) the reduction potentials are F +2.87 V; Cl +1.36; Br +1.09; I +0.54. Here Fe3+ + e− → Fe3+ +0.77.[90] Thus F2, Cl2 and Br2 will oxidize Fe2+ to Fe3+ but Fe3+ will oxidize I− to I2. Iodine has previously been referred to as a moderately strong oxidizing agent.[91] - ↑ The quote marks are not found in the source; they are used here to make it clear that the source employs the word non-metals as a formal term for the subset of chemical elements in question, rather than applying to nonmetals generally.
- ↑ Varying configurations of these nonmetals have been referred to as, for example, basic nonmetals,[98] bioelements,[99] central nonmetals,[100] CHNOPS,[101] essential elements,[102] "non-metals",[103][n 17] orphan nonmetals,[104] or redox nonmetals.[105]
- ↑ Tshitoyan et al. (2019) conducted a machine-based analysis of the proximity of names of the elements based on 3.3 million abstracts published between 1922 and 2018 in more than 1,000 journals. The resulting map shows that "chemically similar elements are seen to cluster together and the overall distribution exhibits a topology reminiscent of the periodic table itself".[107]
- ↑ Jones takes a philosophical or pragmatic view to these questions. He writes: "Though classification is an essential feature of all branches of science, there are always hard cases at the boundaries. The boundary of a class is rarely sharp ... Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics".[112]
- ↑ Metal oxides are usually ionic.[131] On the other hand, oxides of metals with high oxidation states are usually either polymeric or covalent.[132] A polymeric oxide has a linked structure composed of multiple repeating units.[133]
- ↑ Sulfur, an insulator, and selenium, a semiconductor are each photoconductors—their electrical conductivities increase by up to six orders of magnitude when exposed to light.[148]
- ↑ For example, Wulfsberg divides the nonmetals, including B, Si, Ge, As, Sb, Te, Xe, into very electronegative nonmetals (Pauling electronegativity over 2.8) and electronegative nonmetals (1.9 to 2.8). This results in N and O being very electronegative nonmetals, along with the halogens; and H, C, P, S and Se being electronegative nonmetals. Se is further recognized as a semiconducting metalloid.[153]
- ↑ B; Si, Ge; N, P; O, S, Se, Te; halogen nonmetals; and the noble gases.[196]
- ↑ In 2020, high-pressure studies and experiments were said to represent "a very active and vigorous research field".[198]
- ↑ For example, as at April 2023, the commercial price of silicon was $4 per pound or $0.0088 per gram.[206] On the other hand, the price quoted for a 335 gram sample of silicon for hobbyists and science enthusiasts was about $57, or 0.170 per gram, or about 20 times the commercial price.[207]
- ↑ How helium acquired the -ium suffix is explained in the following passage by its discoverer, William Lockyer: "I took upon myself the responsibility of coining the word helium ... I did not know whether the substance ... was a metal like calcium or a gas like hydrogen, but I did know that it behaved like hydrogen [being found in the sun] and that hydrogen, as Dumas had stated, behaved as a metal".[224]
- ↑ Berzelius, who discovered selenium, thought it had the properties of a metal, combined with the properties of sulfur.[228]
- ↑ The Goldhammer-Herzfeld ratio is roughly equal to the cube of the atomic radius divided by the molar volume.[243] More specifically, it is the ratio of the force holding an individual atom's outer electrons in place with the forces on the same electrons from interactions between the atoms in the solid or liquid element. When the interatomic forces are greater than, or equal to, the atomic force, outer electron itinerancy is indicated and metallic behaviour is predicted. Otherwise nonmetallic behaviour is anticipated.[244]
- ↑ (a) The values are from Aylward and Findlay.[270]
(b) Weighable amounts of the extremely radioactive elements At (element 85), Fr (87), and elements with an atomic number higher than Es (99), have not been prepared.[271]
(c) The density values used for At and Fr are theoretical estimates.[272]
(d) Bjerrum classified "heavy metals" as those metals with densities above 7 g/cm3.[273]
(e) Vernon specified a minimum electronegativity of 1.9 for the metalloids, on the revised Pauling scale.[2] - ↑ See also Properties of metals, metalloids and nonmetals, which treats metalloids as a class of their own.
- ↑ Carbon as exfoliated (expanded) graphite,[292] and as carbon nanotube wire;[49] phosphorus as white phosphorus (soft as wax, pliable and can be cut with a knife, at room temperature);[50] sulfur as plastic sulfur;[51] and selenium as selenium wires.[52]
- ↑ Metals have electrical conductivity values of from 6.9×103 S•cm−1 for manganese to 6.3×105 for silver.[294]
- ↑ Metalloids have electrical conductivity values of from 1.5×10−6 S•cm−1 for boron to 3.9×104 for arsenic.[295]
- ↑ Unclassified nonmetals have electrical conductivity values of from ca. 1×10−18 S•cm−1 for the elemental gases to 3±4 in graphite.[296]
- ↑ The halogen nonmetals have electrical conductivity values of from ca. 1×10−18 S•cm−1 for F and Cl to 1.7×10−8 S•cm−1 for iodine.[296][127]
- ↑ The elemental gases have electrical conductivity values of ca. 1×10−18 S•cm−1.[296]
- ↑ They always give "compounds less acidic in character than the corresponding compounds of the [typical] nonmetals".[285]
- ↑ Arsenic trioxide reacts with sulfur trioxide, forming arsenic "sulfate" As2(SO4)3.[307]
- ↑ CO and N2O are "formally the anhydrides of formic and hyponitrous acid, respectively: CO + H2O → H2CO2 (HCOOH, formic acid); N2O + H2O → H2N2O2 (hyponitrous acid)".[311]
- ↑ Disodium helide (Na2He) is a compound of helium and sodium that is stable at high pressures above 113 GPa. Argon forms an alloy with nickel, at 140 GPa and close to 1,500 K however at this pressure argon is no longer a noble gas.[322]
- ↑ Values for the noble gases are from Rahm, Zeng and Hoffmann.[324]
References
Citations
- ↑ 1.0 1.1 1.2 1.3 Larrañaga, Lewis & Lewis 2016, p. 988
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Vernon 2013
- ↑ Hérold 2006, pp. 149–50; Vernon 2020, p. 220
- ↑ Luchinskii & Trifonov 1981, pp. 200–220
- ↑ Jolly 1966, inside cover
- ↑ Rayner-Canham 2020, p. 212
- ↑ 7.0 7.1 7.2 Hein & Arena 2013, pp. 226, G-6
- ↑ Glinka 1958, p. 77; Oxtoby, Gillis & Butler 2015, p. I.23
- ↑ Godovikov & Nenasheva 2020, p. 4; Sanderson 1957, p. 229; Morely & Muir 1892, p. 241
- ↑ 10.0 10.1 Kneen, Rogers & Simpson 1972, pp. 218–219
- ↑ Steudel 2020, p. 43
- ↑ Hill & Holman 2017, p. 162
- ↑ Vernon 2020, p. 220; Rochow 1966, p. 4
- ↑ IUPAC Periodic Table of the Elements
- ↑ Johnson 2007, p. 13
- ↑ Bodner & Pardue 1993, p. 354; Cherim 1971, p. 98
- ↑ Restrepo et al. 2006, p. 411; Thornton & Burdette 2010, p. 86; Hermann, Hoffmann & Ashcroft 2013, pp. 11604‒1‒11604‒5
- ↑ Mewes et al. 2019; Smits et al. 2020; Florez et al. 2022
- ↑ Koenig 1962, p. 108
- ↑ Tidy 1887, pp. 107–108
- ↑ 21.0 21.1 21.2 21.3 Kneen, Rogers & Simpson 1972, pp. 261–264
- ↑ Phillips 1973, p. 7
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 Aylward & Findlay 2008, pp. 6–12
- ↑ 24.0 24.1 Jenkins & Kawamura 1976, p. 88
- ↑ Carapella 1968, p. 30
- ↑ Edwards 2000, pp. 100, 102–103; Herzfeld 1927, pp. 701–705
- ↑ Zumdahl & DeCoste 2010, pp. 455, 456, 469, A40; Earl & Wilford 2021, p. 3-24
- ↑ Still 2016, p. 120
- ↑ Wiberg 2001, pp. 780
- ↑ Wiberg 2001, pp. 824, 785
- ↑ Earl & Wilford 2021, p. 3-24
- ↑ Siekierski & Burgess 2002, p. 86
- ↑ Charlier, Gonze & Michenaud 1994
- ↑ Taniguchi et al. 1984, p. 867: "... black phosphorus ... [is] characterized by the wide valence bands with rather delocalized nature."; Morita 1986, p. 230; Carmalt & Norman 1998, p. 7: "Phosphorus ... should therefore be expected to have some metalloid properties."; Du et al. 2010. Interlayer interactions in black phosphorus, which are attributed to van der Waals-Keesom forces, are thought to contribute to the smaller band gap of the bulk material (calculated 0.19 eV; observed 0.3 eV) as opposed to the larger band gap of a single layer (calculated ~0.75 eV).
- ↑ Wiberg 2001, pp. 742
- ↑ Evans 1966, pp. 124–25
- ↑ Wiberg 2001, pp. 758
- ↑ Stuke 1974, p. 178; Donohue 1982, pp. 386–87; Cotton et al. 1999, p. 501
- ↑ Steudel 1977, p. 240: "... considerable orbital overlap must exist, to form intermolecular, many-center ... [sigma] bonds, spread through the layer and populated with delocalized electrons, reflected in the properties of iodine (lustre, color, moderate electrical conductivity)."; Segal 1989, p. 481: "Iodine exhibits some metallic properties ..."
- ↑ Wiberg 2001, p. 416; Wiberg is here referring to iodine.
- ↑ Elliot 1929, p. 629
- ↑ Fox 2010, p. 31
- ↑ Wibaut 1951, p. 33: "Many substances...are colourless and therefore show no selective absorption in the visible part of the spectrum."
- ↑ Kneen, Rogers & Simpson 1972, pp. 85–86, 237
- ↑ Salinas 2019, p. 379
- ↑ Yang 2004, p. 9
- ↑ Wiberg 2001, pp. 416, 574, 681, 824, 895, 930; Siekierski & Burgess 2002, p. 129
- ↑ Chung 1987; Godfrin & Lauter 1995
- ↑ 49.0 49.1 Janas, Cabrero-Vilatela & Bulmer 2013
- ↑ 50.0 50.1 Faraday 1853, p. 42; Holderness & Berry 1979, p. 255
- ↑ 51.0 51.1 Partington 1944, p. 405
- ↑ 52.0 52.1 Regnault 1853, p. 208
- ↑ Kneen, Rogers & Simpson 1972, pp. 263‒264
- ↑ Langley & Hattori 2014, p. 214
- ↑ 55.0 55.1 Abbott 1966, p. 18
- ↑ Brown et al. 2014, p. 237
- ↑ Lidin 1996, pp. 22, 29; 322, 165; 381, 173–174; 12, 147; 157 [B; P; Se; As; I]; Housecroft & Sharpe 2008, p. 472 [I]
- ↑ Lidin 1996, pp. 52, 58; 386; 140; 361, 365; 372, 376; 403 [C; Si; Ge; S; Sb; Te]; Rochow 1973, p. 1338 [Si]; Sanderson 1967, p. 172 [Ge]; Shkol'nikov 2010, p. 2127 [Sb]; Wiberg 2001, pp. 592 [Te]
- ↑ Matson & Orbaek 2013, p. 85
- ↑ Yoder, Suydam & Snavely 1975, p. 58
- ↑ Young et al. 2018, p. 753
- ↑ Brown et al. 2014, p. 227
- ↑ Siekierski & Burgess 2002, pp. 21, 133, 177
- ↑ Moore 2016; Burford, Passmore & Sanders 1989, p. 54
- ↑ King & Caldwell 1954, p. 17; Brady & Senese 2009, p. 69
- ↑ Chemical Abstracts Service 2021
- ↑ Emsley 2011, pp. 81
- ↑ Cockell 2019, p. 210
- ↑ Scott 2014, p. 3
- ↑ Emsley 2011, p. 184
- ↑ Kneen, Rogers & Simpson 1972, pp. 226, 360
- ↑ Lee 1996, p. 240
- ↑ Greenwood & Earnshaw 2002, p. 43
- ↑ Cressey 2010
- ↑ Siekierski & Burgess 2002, pp. 24–25
- ↑ Siekierski & Burgess 2002, p. 23
- ↑ Petruševski & Cvetković 2018; Grochala 2018
- ↑ Greenwood & Earnshaw 2002, pp. 27, 1232, 1234
- ↑ Siekierski & Burgess 2002, pp. 52, 101, 111, 124, 194
- ↑ Cox 2004, p. 146
- ↑ 81.0 81.1 Vernon 2013, p. 1706
- ↑ Wiberg 2001, passim
- ↑ Vernon 2020, p. 222
- ↑ Atkins & Overton 2010, pp. 377, 389
- ↑ Moody 1991, p. 391
- ↑ Rodgers 2012, p. 504; Wulfsberg 2000, p. 726
- ↑ Stellman 1998, chapter 104–211
- ↑ Nakao 1992, p. 426–427
- ↑ Hill & Holman 2000, p. 196
- ↑ Wiberg 2001, pp. 1761–1762
- ↑ Young 2006, p. 1285
- ↑ Encyclopædia Britannica 2021
- ↑ Royal Society of Chemistry 2021
- ↑ 94.0 94.1 Matson & Orbaek 2013, p. 203
- ↑ Chambers & Holliday 1982, pp. 273–274; Bohlmann 1992, p. 213; Jentzsch 2015, p. 247
- ↑ Kernion 2019, p. 191; Cao et al. 2021, pp. 20–21; Hussain et al. 2023
- ↑ Vassilakis, Kalemos & Mavridis 2014, p. 1; Hanley & Koga 2018, p. 24; Kaiho 2017, ch. 2, p. 1
- ↑ Williams 2007, pp. 1550–1561: H, C, N, P, O, S
- ↑ Wächtershäuser 2014, p. 5: H, C, N, P, O, S, Se
- ↑ Hengeveld & Fedonkin, pp. 181–226: C, N, P, O, S
- ↑ Wakeman 1899, p. 562
- ↑ Fraps 1913, p. 11: H, C, Si, N, P, O, S, Cl
- ↑ Parameswaran at al. 2020, p. 210: H, C, N, P, O, S, Se
- ↑ Knight 2002, p. 148: H, C, N, P, O, S, Se
- ↑ Fraústo da Silva & Williams 2001, p. 500: H, C, N, O, S, Se
- ↑ 106.0 106.1 Bailar et al. 1989, p. 742
- ↑ Tshitoyan et al. 2019, pp. 95–98
- ↑ Russell & Lee 2005, p. 419
- ↑ Hampel & Hawley 1976, p. 174;
- ↑ Goodrich 1844, p. 264; The Chemical News 1897, p. 189; Hampel & Hawley 1976, p. 191; Lewis 1993, p. 835; Hérold 2006, pp. 149–50
- ↑ Tyler 1948, p. 105; Reilly 2002, pp. 5–6
- ↑ 112.0 112.1 Jones 2010, pp. 169–71
- ↑ Stein 1983, p. 165
- ↑ Jolly 1966, p. 20
- ↑ Clugston & Flemming 2000, pp. 100–101, 104–105, 302
- ↑ Maosheng 2020, p. 962
- ↑ Mazej 2020
- ↑ Wiberg 2001, p. 1131
- ↑ Thompson 2004
- ↑ Vernon 2020, p. 229
- ↑ Cox 2000, pp. 258–259; Möller 2003, p. 173; Trenberth & Smith 2005, p. 864
- ↑ Emsley 2011, p. 220
- ↑ Emsley 2011, p. 440
- ↑ Zhu et al. 2014, pp. 644–648
- ↑ Emsley 2011, p. 181
- ↑ Wiberg 2001, pp. 4022
- ↑ 127.0 127.1 Greenwood & Earnshaw 2002, p. 804
- ↑ Rudolph 1973, p. 133: "Oxygen and the halogens in particular ... are therefore strong oxidizing agents."
- ↑ Daniel & Rapp 1976, p. 55
- ↑ 130.0 130.1 Cotton et al. 1999, p. 554
- ↑ Woodward et al. 1999, pp. 133–194
- ↑ Phillips & Williams 1965, pp. 478–479
- ↑ Moeller et al. 2012, p. 314
- ↑ Lanford 1959, p. 176
- ↑ Rayner-Canham 2020, p. 92, 139
- ↑ Massey 2000, p. 113
- ↑ Schmedt, Mangstl & Kraus 2012, p. 7847‒7849
- ↑ Bailar, Moeller & Kleinberg 1965, p. 477; Mee 1964, p. 153
- ↑ Masterton, Hurley & Neth 2011, p. 38
- ↑ McCue 1963, p. 264
- ↑ Dingle 2017, p. 101
- ↑ 142.0 142.1 Cox 1997, pp. 130–132; Emsley 2011, passim
- ↑ Hurlbut 1961, p. 132
- ↑ Emsley 2011, p. 478
- ↑ Greenwood & Earnshaw 2002, p. 277
- ↑ Atkins et al. 2006, p. 320
- ↑ Greenwood & Earnshaw 2002, p. 482; Berger 1997, p. 86
- ↑ Moss 1952, pp. 180, 202
- ↑ 149.0 149.1 149.2 149.3 Cao et al. 2021, pp. 20–21
- ↑ Challoner 2014, p. 5; Government of Canada 2015; Gargaud et al. 2006, p. 447
- ↑ Crichton 2012, p. 6; Scerri 2013; Los Alamos National Laboratory 2021
- ↑ Vernon 2020, p. 218
- ↑ Wulfsberg 2000, pp. 273–274, 620
- ↑ Seese & Daub 1985, p. 65
- ↑ MacKay, MacKay & Henderson 2002, p. 209
- ↑ Cousins, Davidson & García-Vivó 2013, pp. 11809–11811
- ↑ 157.0 157.1 Wiberg 2001, pp. 255–257
- ↑ Liptrot 1983, p. 161
- ↑ Scott & Kanda 1962, p. 153
- ↑ Taylor 1960, p. 316
- ↑ 161.0 161.1 161.2 161.3 161.4 Emsley 2011, passim
- ↑ Crawford 1968, p. 540; Benner, Ricardo & Carrigan 2018, pp. 167—168: "The stability of the carbon—carbon bond ... has made it the first choice element to scaffold biomolecules. Hydrogen is needed for many reasons; at the very least, it terminates C–C chains. Heteroatoms (atoms that are neither carbon nor hydrogen) determine the reactivity of carbon-scaffolded biomolecules. In ... life, these are oxygen, nitrogen and, to a lesser extent, sulfur, phosphorus, selenium, and an occasional halogen."
- ↑ Zhao, Tu & Chan 2021
- ↑ Kosanke et al. 2012, p. 841
- ↑ Wasewar 2021, pp. 322–323
- ↑ Messler 2011, p. 10
- ↑ King et al. 1994, p. 1344; Powell & Tims 1974, pp. 189–191; Cao et al. 2021, pp. 20–21
- ↑ Vernon 2020, pp. 221–223; Rayner-Canham 2020, p. 216
- ↑ Atkins 2001, pp. 24–25
- ↑ National Center for Biotechnology Information 2021
- ↑ Emsley 2011, p. 113
- ↑ Greenwood & Earnshaw 2002, p. 270–271
- ↑ Khan 2001, p. 59
- ↑ Emsley 2011, pp. 376, 380, 640
- ↑ Cox 1997, pp. 130; Emsley 2011, p. 393
- ↑ Cox 1997, pp. 130; Emsley 2011, pp. 515–516, 518
- ↑ Boyd 2011, p. 570
- ↑ Barton 2021, p. 200
- ↑ Shanabrook, Lannin & Hisatsune 1981, pp. 130‒133
- ↑ Borg & Dienes 1992, p. 26
- ↑ Wiberg 2001, p. 796
- ↑ Shang et al. 2021
- ↑ Tang et al. 2021
- ↑ Cacace, de Petris & Troiani 2002, pp. 480‒481
- ↑ Koziel 2002, p. 18
- ↑ Gusmão, Sofer & Pumera 2017, p. 8052–8053; Berger 1997, p. 84; Vernon 2013, pp. 1704‒1705
- ↑ Piro et al. 2006, pp. 1276‒1279
- ↑ Steudel & Eckert 2003, p. 1
- ↑ Greenwood & Earnshaw 2002, pp. 659–660
- ↑ Moss 1952, p. 192; Greenwood & Earnshaw 2002, p. 751
- ↑ Donohue 1982, pp. 48–81
- ↑ Shiell at al. 2021
- ↑ Zhao et al. 2017
- ↑ Donohue 1982, pp. 302–310
- ↑ Brodsky et al. 1972, p. 609–614
- ↑ 196.0 196.1 Keeler & Wothers 2013, p. 293
- ↑ Yousuf 1998, p. 425; Elatresh & Bonev 2020
- ↑ Errandonea 2020, p. 595
- ↑ Su et al. 2020, pp. 1621–1649
- ↑ Nelson 1987, p. 732: crust, atmosphere, hydrosphere; Fortescue 2012, pp. 56, 65: biomass
- ↑ MacKay, MacKay & Henderson 2002, p. 200
- ↑ Cox 1997, pp. 17, 19
- ↑ Ostriker & Steinhardt 2001, pp. 46‒53; Zhu 2020, p. 27
- ↑ Höll et al. 2007
- ↑ U.S. Geological Survey 2023, p. 78
- ↑ U.S. Geological Survey 2023, p. 153
- ↑ Billing Metals & Manufacturing
- ↑ U.S. Geological Survey 2023, p. 72, 86, 147
- ↑ U.S. Geological Survey 2023, pp. 32, 34, 78, 82, 84, 91, 156, 158, 160, 170, 176; Kopteva,Kalimullin & Tcvetkov 2021, p. 1; Oztemel, Salt & Salt 2022, p. 5; Neice & Zornow 2016, p. 1268; Howe-Grant 1995, p. 17; Dalakov, Neuber & Herzog R 2020; Boysen, Cristóbal & Hilbig 2020, pp. 475–489; Gardner & Menon 2018, p. 455; Rajarathnam & Assallo 2016, p. 3; Xia, Ning & Zhu 2020
- ↑ Chand, Kumar & Bhumla 2022, p. 22058
- ↑ Berger 1997, p. 42
- ↑ U.S. Geological Survey 1998
- ↑ 213.0 213.1 Boise State University 2020
- ↑ Hu, Shen & Yu 2017, p. 595
- ↑ Gardner & Menon 2018, p. 454; U.S. Geological Survey 2023, p. 78
- ↑ National Institute of Standards and Technology 2013
- ↑ Emsley 2011, pp. 114–115
- ↑ Emsley 2011, pp. 363–364
- ↑ Emsley 2011, pp. 376–377; 379
- ↑ Emsley 2011, pp. 484–489
- ↑ Emsley 2011, pp. 511, 516–517
- ↑ Bhuwalka et al. 2021, pp. 10097–10107
- ↑ Gaffney & Marley 2017, p. 27
- ↑ Labinger 2019, p. 305
- ↑ Emsley 2011, pp. 42–43, 219–220, 263–264, 341, 441–442, 596, 609
- ↑ Emsley 2011, pp. 84, 128, 180–181, 247
- ↑ Cook 1923, p. 124
- ↑ Weeks 1945, p. 161
- ↑ Emsley 2011, pp. 113, 363, 378, 477, 514–515
- ↑ Weeks 1945, p. 22; Emsley 2011, p. 40
- ↑ Klein 1994, p. 168
- ↑ Lidin 1996, pp. 64‒65
- ↑ de L'Aunay 1566, p. 7
- ↑ Homberg 1708, p. 350; vide Kim 2000
- ↑ de Clave 1641
- ↑ Schlager & Lauer 2000, p. 370
- ↑ Strathern 2000, p. 239
- ↑ Criswell p. 1140
- ↑ Salzberg 1991, p. 204
- ↑ Kendall 1811, pp. 298–303
- ↑ Brande 1821, p. 5
- ↑ Edwards & Sienko 1983, pp. 691–96
- ↑ Edwards & Sienko 1983, p. 693
- ↑ Herzfeld 1927; Edwards 2000, pp. 100–03
- ↑ Kubaschewski 1949, pp. 931–940
- ↑ Remy 1956, p. 9
- ↑ White 1962, p. 106: It makes a ringing sound when struck.
- ↑ Johnson 1966, pp. 3–4
- ↑ Horvath 1973, pp. 335–336
- ↑ Rao & Ganguly 1986
- ↑ Smith & Dwyer 1991, p. 65: The difference between melting point and boiling point.
- ↑ 252.0 252.1 Herman 1999, p. 702
- ↑ Hill, Holman & Hulme 2017, p. 182: Atomic conductance is the electrical conductivity of one mole of a substance. It is equal to electrical conductivity divided by molar volume.
- ↑ Suresh & Koga 2001, pp. 5940–5944
- ↑ Johnson 2007, pp. 15–16
- ↑ 256.0 256.1 Edwards 2010, pp. 941–965
- ↑ Povh & Rosin 2017, p. 131
- ↑ Beach 1911
- ↑ Stott 1956, pp. 100–102
- ↑ Parish 1977, p. 178
- ↑ Sanderson 1957, p. 229
- ↑ Hare & Bache 1836, p. 310
- ↑ Chambers 1743: "That which distinguishes metals from all other bodies ... is their heaviness ..."
- ↑ Edwards 2000, p. 85
- ↑ Russell & Lee 2005, p. 466
- ↑ Atkins et al. 2006, pp. 320–21
- ↑ Zhigal'skii & Jones 2003, p. 66
- ↑ Emsley 1971, p. 1
- ↑ Jones 2010, p. 169
- ↑ Aylward & Findlay 2008, pp. 6–13; 126
- ↑ Edelstein & Morrs 2009, p. 123
- ↑ Arblaster JW (ed.) 2018, p. 269; Lavrukhina & Pozdnyakov 1970, p. 269
- ↑ Bjerrum N 1936
- ↑ Johnson 1966, pp. 3–5, 15
- ↑ Oderberg 2007, p. 97
- ↑ Bertomeu-Sánchez, Garcia-Belmar & Bensaude-Vincent 2002, pp. 248–249
- ↑ Dupasquier 1844, pp. 66–67
- ↑ Berzelius 1832, pp. 248–276
- ↑ The Chemical News 1864, p. 22
- ↑ Renouf 1901, pp. 268
- ↑ Vernon 2020, pp. 217–225
- ↑ Tregarthen 2003, p. 10
- ↑ Lewis 1993, pp. 28, 827
- ↑ Lewis 1993, pp. 28, 813
- ↑ 285.0 285.1 285.2 Rochow 1966, p. 4
- ↑ Wiberg 2001, p. 780; Emsley 2011, p. 397; Rochow 1966, pp. 23, 84
- ↑ Kneen, Rogers & Simpson 1972, pp. 321, 404, 436
- ↑ Kneen, Rogers & Simpson 1972, p. 439
- ↑ Kneen, Rogers & Simpson 1972, p. 465
- ↑ Kneen, Rogers & Simpson 1972, p. 308
- ↑ Wiberg 2001, pp. 505, 681, 781; Glinka 1958, p. 355
- ↑ Chung 1987, pp. 4190‒4198; Godfrin & Lauter 1995, pp. 216‒218
- ↑ Wiberg 2001, p. 416
- ↑ Desai, James & Ho 1984, p. 1160; Matula 1979, p. 1260
- ↑ Schaefer 1968, p. 76; Carapella 1968, pp. 29‒32
- ↑ 296.0 296.1 296.2 Bogoroditskii & Pasynkov 1967, p. 77; Jenkins & Kawamura 1976, p. 88
- ↑ Kneen, Rogers & Simpson 1972, p. 264
- ↑ Rayner-Canham 2018, p. 203
- ↑ Welcher 2001, p. 3–32: "The elements change from ... metalloids, to moderately active nonmetals, to very active nonmetals, and to a noble gas."
- ↑ Mackin 2014, p. 80
- ↑ Johnson 1966, pp. 105–108
- ↑ Stein 1969, pp. 5396‒5397; Pitzer 1975, pp. 760‒761
- ↑ Porterfield 1993, p. 336
- ↑ 304.0 304.1 Rao 2002, p. 22
- ↑ Wells 1984, p. 534
- ↑ Rochow 1966, p. 4; Atkins et al. 2006, pp. 8, 122–123
- ↑ Wiberg 2001, p. 750
- ↑ Sidorov 1960, pp. 599‒603
- ↑ 309.0 309.1 309.2 309.3 Puddephatt & Monaghan 1989, p. 59
- ↑ Sanderson 1967, p. 172; Mingos 2019, p. 27
- ↑ House 2008, p. 441
- ↑ McMillan 2006, p. 823
- ↑ Mingos 2019, p. 27; Sanderson 1967, p. 172
- ↑ King 1995, p. 182
- ↑ Wiberg 2001, p. 399
- ↑ Kläning & Appelman 1988, p. 3760
- ↑ Ritter 2011, p. 10
- ↑ Yamaguchi & Shirai 1996, p. 3
- ↑ Vernon 2020, p. 223
- ↑ Vernon 2020, p. 220
- ↑ Woodward et al. 1999, p. 134
- ↑ Dalton 2019
- ↑ Aylward & Findlay 2008, p. 132
- ↑ Rahm, Zeng & Hoffmann 2019, p. 345
- ↑ Aylward & Findlay 2008, p. 126
- ↑ Steudel 1977, p. 176
Bibliography
- Abbott D 1966, An Introduction to the Periodic Table, J. M. Dent & Sons, London
- Arblaster JW (ed.) 2018, Selected Values of the Crystallographic Properties of Elements, ASM International, Materials Park, Ohio, ISBN 978-1-62708-154-2
- Atkins PA 2001, The Periodic Kingdom: A Journey Into the Land of the Chemical Elements, Phoenix, London, ISBN:978-1-85799-449-0
- Atkins PA et al. 2006, Shriver & Atkins' Inorganic Chemistry, 4th ed., Oxford University Press, Oxford, ISBN:978-0-7167-4878-6
- Atkins PA & Overton T 2010, Shriver & Atkins' Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, ISBN:978-0-19-923617-6
- Aylward G and Findlay T 2008, SI Chemical Data, 6th ed., John Wiley & Sons Australia, Milton, ISBN 978-0-470-81638-7
- Bailar JC, Moeller T & Kleinberg J 1965, University Chemistry, DC Heath, Boston
- Bailar JC et al. 1989, Chemistry, 3rd ed., Harcourt Brace Jovanovich, San Diego, ISBN:978-0-15-506456-0
- Barton AFM 2021, States of Matter, States of Mind, CRC Press, Boca Raton, ISBN 978-0-7503-0418-4
- Beach FC (ed.) 1911, The Americana: A universal reference library, vol. XIII, Mel–New, Metalloid, Scientific American Compiling Department, New York
- Benner SA, Ricardo A & Carrigan MA 2018, "Is there a common chemical model for life in the universe?", in Cleland CE & Bedau MA (eds.), The Nature of Life: Classical and Contemporary Perspectives from Philosophy and Science, Cambridge University Press, Cambridge, ISBN 978-1-108-72206-3
- Berger LI 1997, Semiconductor Materials, CRC Press, Boca Raton, ISBN 978-0-8493-8912-2
- Bertomeu-Sánchez JR, Garcia-Belmar A & Bensaude-Vincent B 2002, "Looking for an order of things: Textbooks and chemical classifications in nineteenth century France", Ambix, vol. 49, no. 3, doi:10.1179/amb.2002.49.3.227
- Berzelius JJ & Bache AD 1832, "An essay on chemical nomenclature, prefixed to the treatise on chemistry", The American Journal of Science and Arts, vol. 22
- Billing Metals & Manufacturing, Silicon, Large Collectors sample. Element 14., accessed May 2, 2023
- Bjerrum N 1936, Bjerrum's Inorganic Chemistry, Heinemann, London
- Bodner GM & Pardue HL 1993, Chemistry, An Experimental Science, John Wiley & Sons, New York, ISBN:0-471-59386-9
- Bogoroditskii NP & Pasynkov VV 1967, Radio and Electronic Materials, Iliffe Books, London
- Bohlmann R 1992, "Synthesis of halides", in Winterfeldt E (ed.), Heteroatom manipulation, Pergamon Press, Oxford, ISBN 978-0-08-091249-3
- Boise State University 2020, "Cost-effective manufacturing methods breathe new life into black phosphorus research", Micron School of Materials Science and Engineering, accessed July 9, 2021
- Borg RG & Dienes GJ 1992, The Physical Chemistry of Solids, Academic Press, Boston, ISBN 978-0-12-118420-9
- Boyd R 2011, "Selenium stories", Nature Chemistry, vol. 3, doi:10.1038/nchem.1076
- Boysen B, Cristóbal J & Hilbig J 2020, "Economic and environmental assessment of water reuse in industrial parks: case study based on a Model Industrial Park", Journal of Water Reuse and Desalination, vol. 10, no. 4, pp. 475–489, doi: 10.2166/wrd.2020.034
- Brady JE & Senese F 2009, Chemistry: The study of Matter and its Changes, 5th ed., John Wiley & Sons, New York, ISBN:978-0-470-57642-7
- Brande WT 1821, A Manual of Chemistry, vol. II, John Murray, London
- Brodsky MH, Gambino RJ, Smith JE Jr & Yacoby Y 1972, "The Raman spectrum of amorphous tellurium", Physica Status Solidi B, vol. 52, doi:10.1002/pssb.2220520229
- Brown TL et al. 2014, Chemistry: The Central Science, 3rd ed., Pearson Australia: Sydney, ISBN 978-1-4425-5460-3
- Burford N, Passmore J & Sanders JCP 1989, "The preparation, structure, and energetics of homopolyatomic cations of groups 16 (the chalcogens) and 17 (the halogens)", in Liebman JF & Greenberg A (eds.), From atoms to polymers: isoelectronic analogies, VCH, New York, ISBN 978-0-89573-711-3
- Cacace F, de Petris G & Troiani A 2002, "Experimental detection of tetranitrogen", Science, vol. 295, no. 5554, doi:10.1126/science.1067681
- Cao C et al. 2021, "Understanding periodic and non-periodic chemistry in periodic tables", Frontiers in Chemistry, vol. 8, no. 813, doi:10.3389/fchem.2020.00813
- Carapella SC 1968, "Arsenic" in Hampel CA (ed.), The Encyclopedia of the Chemical Elements, Reinhold, New York
- Carmalt CJ & Norman NC 1998, 'Arsenic, antimony and bismuth: Some general properties and aspects of periodicity', in Norman NC (ed.), Chemistry of Arsenic, Antimony and Bismuth, Blackie Academic & Professional, London, pp. 1–38, ISBN:0-7514-0389-X
- Challoner J 2014, The Elements: The New Guide to the Building Blocks of our Universe, Carlton Publishing Group, ISBN:978-0-233-00436-5
- Chambers E 1743, in "Metal", Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.), vol. 2, D Midwinter, London
- Chambers C & Holliday AK 1982, Inorganic Chemistry, Butterworth & Co., London, ISBN 978-0-408-10822-5
- Chand H, Kumar A & Bhumla P 2022, "Scalable production of ultrathin boron nanosheets from a low-cost precursor", Advanced Materials Interfaces, vol. 9, no. 2, doi:10.1002/admi.202200508
- Charlier J-C, Gonze X, Michenaud J-P 1994, First-principles study of the stacking effect on the electronic properties of graphite(s), Carbon, vol. 32, no. 2, pp. 289–99, doi:10.1016/0008-6223(94)90192-9
- Chemical Abstracts Service 2021, CAS REGISTRY database as of November 2, Case #01271182
- Cherim SM 1971, Chemistry for Laboratory Technicians, Saunders, Philadelphia, ISBN 978-0-7216-2515-7
- Chung DD 1987, "Review of exfoliated graphite", Journal of Materials Science, vol. 22, doi:10.1007/BF01132008
- Clugston MJ & Flemming R 2000, Advanced Chemistry, Oxford University Press, Oxford, ISBN:978-0-19-914633-8
- Cockell C 2019, The Equations of Life: How Physics Shapes Evolution, Atlantic Books, London, ISBN:978-1-78649-304-0
- Cook CG 1923, Chemistry in Everyday Life: With Laboratory Manual, D Appleton, New York
- Cotton A et al. 1999, Advanced Inorganic Chemistry, 6th ed., Wiley, New York, ISBN:978-0-471-19957-1
- Cousins DM, Davidson MG & García-Vivó D 2013, "Unprecedented participation of a four-coordinate hydrogen atom in the cubane core of lithium and sodium phenolates", Chemical Communications, vol. 49, doi:10.1039/C3CC47393G
- Cox AN (ed.) 2000, Allen's Astrophysical Quantities, 4th ed., AIP Press, New York, ISBN 978-0-387-98746-0
- Cox PA 1997, The Elements: Their Origins, Abundance, and Distribution, Oxford University Press, Oxford, ISBN:978-0-19-855298-7
- Cox T 2004, Inorganic Chemistry, 2nd ed., BIOS Scientific Publishers, London, ISBN:978-1-85996-289-3
- Crawford FH 1968, Introduction to the Science of Physics, Harcourt, Brace & World, New York
- Crichton R 2012, Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function, 2nd ed., Elsevier, Amsterdam, ISBN 978-0-444-53783-6
- Cressey D 2010, "Chemists re-define hydrogen bond", Nature newsblog, accessed August 23, 2017
- Criswell B 2007, "Mistake of having students be Mendeleev for just a day", Journal of Chemical Education, vol. 84, no. 7, pp. 1140–1144, doi:10.1021/ed084p1140
- Dalakov P, Neuber E & Herzog R 2020, "Innovative neon refrigeration unit operating down to 30 K", MATEC Web of Conferences, vol. 324, doi: 10.1051/matecconf/202032401003
- Dalton L 2019, "Argon reacts with nickel under pressure-cooker conditions", Chemical & Engineering News, accessed November 6, 2019
- Daniel PL & Rapp RA 1976, "Halogen corrosion of metals", in Fontana MG & Staehle RW (eds.), Advances in Corrosion Science and Technology, Springer, Boston, doi:10.1007/978-1-4615-9062-0_2
- de Clave E 1641, New Philosophical Light of True Principles and Elements of Nature, Olivier Devarennes, Paris, accessed February 24, 2022
- de L'Aunay L 1566, Responce au discours de maistre Iacques Grevin, docteur de Paris, qu'il a escript contre le livre de maistre Loys de l'Aunay, medecin en la Rochelle, touchant la faculté de l'antimoine (Response to the Speech of Master Jacques Grévin,... Which He Wrote Against the Book of Master Loys de L'Aunay,... Touching the Faculty of Antimony), De l'Imprimerie de Barthelemi Berton, La Rochelle
- Desai PD, James HM & Ho CY 1984, "Electrical resistivity of aluminum and manganese", Journal of Physical and Chemical Reference Data, vol. 13, no. 4, doi:10.1063/1.555725
- Dingle A 2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton, ISBN 978-0-85762-505-2
- Donohue J 1982, The Structures of the Elements, Robert E. Krieger, Malabar, Florida, ISBN:978-0-89874-230-5
- Du Y, Ouyang C, Shi S & Lei M 2010, "Ab initio studies on atomic and electronic structures of black phosphorus", Journal of Applied Physics, vol. 107, no. 9, pp. 093718–1–4, doi:10.1063/1.3386509
- Dupasquier A 1844, Traité élémentaire de chimie industrielle, Charles Savy Juene, Lyon
- Earl B & Wilford D 2021, Cambridge O Level Chemistry, Hodder Education, London, ISBN:978-1-3983-1059-9
- Edelstein NM & Morrs LR 2009, "Chemistry of the actinide elements", in Nagy S (ed.), Radiochemistry and Nuclear Chemistry: Volume II, Encyclopedia of Life Support Systems, EOLSS Publishers, Oxford, pp. 118–176, ISBN 978-1-84826-577-6
- Edwards PP 2000, "What, why and when is a metal?", in Hall N (ed.), The New Chemistry, Cambridge University, Cambridge, pp. 85–114, ISBN:978-0-521-45224-3
- Edwards PP et al. 2010, "... a metal conducts and a non-metal doesn’t", Philosophical Transactions of the Royal Society A, 2010, vol, 368, no. 1914, doi:10.1098/rsta.2009.0282
- Edwards PP & Sienko MJ 1983, "On the occurrence of metallic character in the periodic table of the elements", Journal of Chemical Education, vol. 60, no. 9, doi:10.1021/ed060p691, PMID 25666074
- Elatresh SF & Bonev SA 2020, "Stability and metallization of solid oxygen at high pressure", Physical Chemistry Chemical Physics, vol. 22, no. 22, doi:10.1039/C9CP05267D
- Elliot A 1929, "The absorption band spectrum of chlorine", Proceedings of the Royal Society A, vol. 123, no. 792, pp. 629–644, doi:10.1098/rspa.1929.0088
- Emsley J 1971, The Inorganic Chemistry of the Non-metals, Methuen Educational, London, ISBN:978-0-423-86120-4
- Emsley J 2011, Nature's Building Blocks: An A–Z Guide to the Elements, Oxford University Press, Oxford, ISBN:978-0-19-850341-5
- Encyclopædia Britannica 2021, Periodic table, accessed September 21, 2021
- Errandonea D 2020, "Pressure-induced phase transformations", Crystals, vol. 10, doi:10.3390/cryst10070595
- Evans RC 1966, An Introduction to Crystal Chemistry, 2nd ed., Cambridge University, Cambridge
- Faraday M 1853, The Subject Matter of a Course of Six Lectures on the Non-metallic Elements, (arranged by John Scoffern), Longman, Brown, Green, and Longmans, London
- Florez et al. 2022, From the gas phase to the solid state: The chemical bonding in the superheavy element flerovium, The Journal of Chemical Physics, vol. 157, 064304, doi:10.1063/5.0097642
- Fortescue JAC 2012, Environmental Geochemistry: A Holistic Approach, Springer-Verlag, New York, ISBN 978-1-4612-6047-9
- Fox M 2010, Optical Properties of Solids, 2nd ed., Oxford University Press, New York, ISBN 978-0-19-957336-3
- Fraps GS 1913, Principles of Agricultural Chemistry, The Chemical Publishing Company, Easton, PA
- Fraústo da Silva JJR & Williams RJP 2001, The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed., Oxford University Press, Oxford, ISBN 978-0-19-850848-9
- Gaffney J & Marley N 2017, General Chemistry for Engineers, Elsevier, Amsterdam, ISBN 978-0-12-810444-6
- Gardner AJ & Menon DK 2018, "Moving to human trials for argon neuroprotection in neurological injury: A narrative review", British Journal of Anaesthesia, vol. 120, no. 4, pp. 453–468, doi: 10.1016/j.bja.2017.10.017
- Gargaud M et al. (eds.) 2006, Lectures in Astrobiology, vol. 1, part 1: The Early Earth and Other Cosmic Habitats for Life, Springer, Berlin, ISBN:978-3-540-29005-6
- Glinka N 1958, General chemistry, Sobolev D (trans.), Foreign Languages Publishing House, Moscow
- Godfrin H & Lauter HJ 1995, "Experimental properties of 3He adsorbed on graphite", in Halperin WP (ed.), Progress in Low Temperature Physics, volume 14, Elsevier Science B.V., Amsterdam, ISBN:978-0-08-053993-5
- Godovikov AA & Nenasheva N 2020, Structural-chemical Systematics of Minerals, 3rd ed., Springer, Cham, Switzerland, ISBN 978-3-319-72877-3
- Goodrich BG 1844, A Glance at the Physical Sciences, Bradbury, Soden & Co., Boston
- Government of Canada 2015, Periodic table of the elements, accessed August 30, 2015
- Greenwood NN & Earnshaw A 2002, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, ISBN:978-0-7506-3365-9
- Grochala W 2018, "On the position of helium and neon in the Periodic Table of Elements", Foundations of Chemistry, vol. 20, pp. 191–207, doi:10.1007/s10698-017-9302-7
- Gusmão R, Sofer Z & Pumera M 2017, "Black phosphorus rediscovered: From bulk material to monolayers", Angewandte Chemie International Edition, vol. 56, no. 28, doi:10.1002/anie.201610512
- Hampel CA & Hawley GG 1976, Glossary of Chemical Terms, Van Nostrand Reinhold, New York, ISBN:978-0-442-23238-2
- Hanley JJ & Koga KT 2018, "Halogens in terrestrial and cosmic geochemical systems: Abundances, geochemical behaviours, and analytical methods" in The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle, Harlov DE & Aranovich L (eds.), Springer, Cham, ISBN 978-3-319-61667-4
- Hare RA & Bache F 1836, Compendium of the Course of Chemical Instruction in the Medical Department of the University of Pennsylvania, 3rd ed., JG Auner, Philadelphia
- Hein M & Arena S 2013, Foundations of College Chemistry, John Wiley & Sons, ISBN 978-1-118-29823-7
- Hengeveld R & Fedonkin MA 2007, "Bootstrapping the energy flow in the beginning of life", Acta Biotheoretica, vol. 55, doi:10.1007/s10441-007-9019-4
- Herman ZS 1999, "The nature of the chemical bond in metals, alloys, and intermetallic compounds, according to Linus Pauling", in Maksić, ZB, Orville-Thomas WJ (eds.), 1999, Pauling's Legacy: Modern Modelling of the Chemical Bond, Elsevier, Amsterdam, doi:10.1016/S1380-7323(99)80030-2
- Hermann A, Hoffmann R & Ashcroft NW 2013, "Condensed astatine: Monatomic and metallic", Physical Review Letters, vol. 111, doi:10.1103/PhysRevLett.111.116404
- Hérold A 2006, "An arrangement of the chemical elements in several classes inside the periodic table according to their common properties", Comptes Rendus Chimie, vol. 9, no. 1, doi:10.1016/j.crci.2005.10.002
- Herzfeld K 1927, "On atomic properties which make an element a metal", Physical Review, vol. 29, no. 5, doi:10.1103/PhysRev.29.701
- Hill G & Holman J 2000, Chemistry in Context, 5th ed., Nelson Thornes, Cheltenham, ISBN:0-17-448307-4
- Hill G, Holman J & Hulme PG 2017, Chemistry in Context, 7th ed., Oxford University Press, Oxford, ISBN 978-0-19-839618-5
- Holderness A & Berry M 1979, Advanced Level Inorganic Chemistry, 3rd ed., Heinemann Educational Books, London, ISBN:978-0-435-65435-1
- Höll, Kling & Schroll E 2007, "Metallogenesis of germanium—A review", Ore Geology Reviews, vol. 30, nos. 3–4, pp. 145–180, doi:10.1016/j.oregeorev.2005.07.034
- Homberg W 1708, "Des essais de chimie", in Histoire De L'Academie Royale Des Sciences: Avec les Memoires de Mathematique & de Physique, L'Académie, Paris
- Horvath AL 1973, "Critical temperature of elements and the periodic system", Journal of Chemical Education, vol. 50, no. 5, doi:10.1021/ed050p335
- House JE 2008, Inorganic Chemistry, Elsevier, Amsterdam, ISBN:978-0-12-356786-4
- Housecroft CE & Sharpe AG 2008, Inorganic Chemistry, 3rd ed., Prentice-Hall, Harlow, ISBN 978-0-13-175553-6
- Howe-Grant MI (ed.) 1995, Fluorine Chemistry: A Comprehensive Treatment, John Wiley and Sons, New York, p. 17, ISBN 978-0-471-12031-5
- Hu Z, Shen Z & Yu JC 2017, "Phosphorus containing materials for photocatalytic hydrogen evolution", Green Chemistry, vol. 19, no. 3, pp. 588–613, doi:10.1039/C6GC02825J
- Hurlbut Jr CS 1961, Manual of Mineralogy, 15th ed., John Wiley & Sons, New York
- Hussain et al. 2023, "Tuning the electronic properties of molybdenum di-sulphide monolayers via doping using first-principles calculations", Physica Scripta, vol. 98, no. 2, doi:10.1088/1402-4896/acacd1
- IUPAC Periodic Table of the Elements, accessed October 11, 2021
- Janas D, Cabrero-Vilatela, A & Bulmer J 2013, "Carbon nanotube wires for high-temperature performance", Carbon, vol. 64, pp. 305–314, doi:10.1016/j.carbon.2013.07.067
- Jenkins GM & Kawamura K 1976, Polymeric Carbons—Carbon Fibre, Glass and Char, Cambridge University Press, Cambridge, ISBN:978-0-521-20693-8
- Jentzsch AV & Matile S 2015, "Anion transport with halogen bonds", in Metrangolo P & Resnati G (eds.), Halogen Bonding I: Impact on Materials Chemistry and Life Sciences, Springer, Cham, ISBN 978-3-319-14057-5
- Johnson D (ed.) 2007, Metals and Chemical Change, RSC Publishing, Cambridge, ISBN 978-0-85404-665-2
- Johnson RC 1966, Introductory Descriptive Chemistry, WA Benjamin, New York
- Jolly WL 1966, The Chemistry of the Non-metals, Prentice-Hall, Englewood Cliffs, New Jersey
- Jones BW 2010, Pluto: Sentinel of the Outer Solar System, Cambridge University, Cambridge, ISBN:978-0-521-19436-5
- Kaiho T 2017, Iodine Made Simple, CRC Press, e-book, doi:10.1201/9781315158310
- Keeler J & Wothers P 2013, Chemical Structure and Reactivity: An Integrated Approach, Oxford University Press, Oxford, ISBN 978-0-19-960413-5
- Kendall EA 1811, Pocket Encyclopædia, 2nd ed., vol. III, Longman, Hurst, Rees, Orme, and Co., London
- Kernion MC & Mascetta JA 2019, Chemistry: The Easy Way, 6th ed., Kaplan, New York, ISBN 978-1-4380-1210-0
- Khan N 2001, An Introduction to Physical Geography, Concept Publishing, New Delhi, ISBN:978-81-7022-898-1
- Kim MG 2000, "Chemical analysis and the domains of reality: Wilhelm Homberg's Essais de chimie, 1702–1709", Studies in History and Philosophy of Science Part A, vol. 31, no. 1, pp. 37–69, doi:10.1016/S0039-3681(99)00033-3
- King RB 1994, Encyclopedia of Inorganic Chemistry, vol. 3, John Wiley & Sons, New York, ISBN 978-0-471-93620-6
- King RB 1995, Inorganic Chemistry of Main Group Elements, VCH, New York, ISBN:978-1-56081-679-9
- King GB & Caldwell WE 1954, The Fundamentals of College Chemistry, American Book Company, New York
- Kläning UK & Appelman EH 1988, "Protolytic properties of perxenic acid", Inorganic Chemistry, vol. 27, no. 21, doi:10.1021/ic00294a018
- Klein U 1994, "Origin of the concept of chemical compound", Science in Context, no. 7, vol. 2, pp. 163–204, doi:10.1017/s0269889700001666
- Kneen WR, Rogers MJW & Simpson P 1972, Chemistry: Facts, Patterns, and Principles, Addison-Wesley, London, ISBN:978-0-201-03779-1
- Knight J 2002, Science of Everyday Things: Real-life chemistry, Gale Group, Detroit, ISBN 9780787656324
- Koenig SH 1962, in Proceedings of the International Conference on the Physics of Semiconductors, held at Exeter, July 16−20, 1962, The Institute of Physics and the Physical Society, London
- Kopteva A, Kalimullin L & Tcvetkov P 2021, "Prospects and obstacles for green hydrogen production in Russia", Energies, vol. 14, no. 3, pp. 1–21, doi:10.3390/en14030718
- Kosanke et al. 2012, Encyclopedic Dictionary of Pyrotechnics (and Related Subjects), Part 3 – P to Z, Pyrotechnic Reference Series No. 5, Journal of Pyrotechnics, Whitewater, Colorado, ISBN:978-1-889526-21-8
- Koziel JA 2002, "Sampling and sample preparation for indoor air analysis", in Pawliszyn J (ed.), Comprehensive Analytical Chemistry, vol. 37, Elsevier Science B.V., Amsterdam, ISBN:978-0-444-50510-1
- Kubaschewski O 1949, "The change of entropy, volume and binding state of the elements on melting", Transactions of the Faraday Society, vol. 45, doi:10.1039/TF9494500931
- Labinger JA 2019, "The history (and pre-history) of the discovery and chemistry of the noble gases", in Giunta CJ, Mainz VV & Girolami GS (eds.), 150 Years of the Periodic Table: A Commemorative Symposium, Springer Nature, Cham, Switzerland, ISBN:978-3-030-67910-1
- Lanford OE 1959, Using Chemistry, McGraw-Hill, New York
- Langley RH & Hattori H 2014, 1,001 Practice Problems: Chemistry For Dummies, John Wiley & Sons, Hoboken, NJ, ISBN 978-1-118-54932-2
- Larrañaga MD, Lewis RJ & Lewis RA 2016, Hawley's Condensed Chemical Dictionary, 16th ed., Wiley, Hoboken, New York, ISBN 978-1-118-13515-0
- Lavrukhina AK & Pozdnyakov AA 1970, Analytical Chemistry of Technetium, Promethium, Astatine, and Francium, R Kondor, trans., Ann Arbor–Humphrey Science Publishers, Ann Arbor, ISBN 978-0-250-39923-9
- Lee JD 1996, Concise Inorganic Chemistry, 5th ed., Blackwell Science, Oxford, ISBN:978-0-632-05293-6
- Lewis RJ 1993, Hawley's Condensed Chemical Dictionary, 12th ed., Van Nostrand Reinhold, New York, ISBN:978-0-442-01131-4
- Lidin RA 1996, Inorganic Substances Handbook, Begell House, New York, ISBN:978-0-8493-0485-9
- Liptrot GF 1983, Modern Inorganic Chemistry, 4th ed., Bell & Hyman, ISBN 978-0-7135-1357-8
- Los Alamos National Laboratory 2021, Periodic Table of Elements: A Resource for Elementary, Middle School, and High School Students, accessed September 19, 2021
- Luchinskii GP & Trifonov DN 1981, "Some problems of chemical elements classification and the structure of the periodic system", in Uchenie o Periodichnosti. Istoriya i Sovremennoct, (Russian) Nauka, Moscow
- MacKay KM, MacKay RA & Henderson W 2002, Introduction to Modern Inorganic Chemistry, 6th ed., Nelson Thornes, Cheltenham, ISBN:978-0-7487-6420-4
- Mackin M 2014, Study Guide to Accompany Basics for Chemistry, Elsevier Science, Saint Louis, ISBN:978-0-323-14652-4
- Maosheng M 2020, "Noble gases in solid compounds show a rich display of chemistry with enough pressure", Frontiers in Chemistry, vol. 8, doi:10.3389/fchem.2020.570492
- Massey AG 2000, Main Group Chemistry, 2nd ed., John Wiley & Sons, Chichester, ISBN 978-0-471-49039-5
- Masterton W, Hurley C & Neth E 2011, Chemistry: Principles and Reactions, 7th ed., Brooks/Cole, Belmont, California, ISBN:978-1-111-42710-8
- Matson M & Orbaek AW 2013, Inorganic Chemistry for Dummies, John Wiley & Sons: Hoboken, ISBN 978-1-118-21794-8
- Matula RA 1979, "Electrical resistivity of copper, gold, palladium, and silver", Journal of Physical and Chemical Reference Data, vol. 8, no. 4, doi:10.1063/1.555614
- Mazej Z 2020, "Noble-gas chemistry more than half a century after the first report of the noble-gas compound", Molecules, vol. 25, no. 13, doi:10.3390/molecules25133014, PMID 32630333, PMC 7412050
- McCue JJ 1963, World of Atoms: An Introduction to Physical Science, Ronald Press, New York
- McMillan P 2006, "A glass of carbon dioxide", Nature, vol. 441, doi:10.1038/441823a
- Mee AJ 1964, Physical Chemistry, Aldine Publishing, Chicago
- Messler Jr RW 2011, The Essence of Materials for Engineers, Jones and Bartlett Learning, Sudbury, Massachusetts, ISBN 978-0-7637-7833-0
- Mewes et al. 2019, Copernicium: A relativistic noble liquid, Angewandte Chemie International Edition, vol. 58, pp. 17964–17968, doi:10.1002/anie.201906966
- Mingos DMP 2019, "The discovery of the elements in the Periodic Table", in Mingos DMP (ed.), The Periodic Table I. Structure and Bonding, Springer Nature, Cham, doi:10.1007/978-3-030-40025-5
- Moeller T et al. 2012, Chemistry: With Inorganic Qualitative Analysis, Academic Press, New York, ISBN 978-0-12-503350-3
- Möller D 2003, Luft: Chemie, Physik, Biologie, Reinhaltung, Recht, Walter de Gruyter, Berlin, ISBN:978-3-11-016431-2
- Moody B 1991, Comparative Inorganic Chemistry, 3rd ed., Edward Arnold, London, ISBN:978-0-7131-3679-1
- Moore JT 2016, Chemistry for Dummies, 2nd ed., ch. 16, Tracking periodic trends, John Wiley & Sons: Hoboken, ISBN 978-1-119-29728-4
- Morita A 1986, 'Semiconducting black phosphorus', Journal of Applied Physics A, vol. 39, no. 4, pp. 227–42, doi:10.1007/BF00617267
- Morely HF & Muir MM 1892, Watt's Dictionary of Chemistry, vol. 3, Longman's Green, and Co., London
- Moss, TS 1952, Photoconductivity in the Elements, Butterworths Scientific, London
- Nakao Y 1992, "Dissolution of noble metals in halogen–halide–polar organic solvent systems", Journal of the Chemical Society, Chemical Communications, no. 5, doi:10.1039/C39920000426
- National Center for Biotechnology Information 2021, "PubChem compound summary for CID 402, Hydrogen sulfide", accessed August 31, 2021
- National Institute of Standards and Technology 2013, SRM 4972 – Radon-222 Emanation Standard, accessed August 1, 2021
- Neice AE & Zornow MH 2016, "Editorial: Xenon anaesthesia for all, or only a select few?", Anaesthesia, vol. 71, no. 11, pp. 1259–1272 (1268), doi: 10.1111/anae.13569
- Nelson PG 1987, "Important elements", Journal of Chemical Education, vol. 68, no. 9, doi:10.1021/ed068p732
- Oderberg DS 2007, Real Essentialism, Routledge, New York, ISBN:978-1-134-34885-5
- Ostriker JP & Steinhardt PJ 2001, "The quintessential universe", Scientific American, vol. 284, no. 1, pp. 46–53 PMID 11132422, doi: 10.1038/scientificamerican0101-46
- Oxtoby DW, Gillis HP & Butler LJ 2015, Principles of Modern Chemistry, 8th ed., Cengage Learning, Boston, ISBN:978-1-305-07911-3
- Oztemel BH, Salt I, Salt Y 2022, "Carbon dioxide utilization: Process simulation of synthetic fuel production from flue gases", Chemical Industry and Chemical Engineering Quarterly, vol. 28, no. 4, doi:10.2298/CICEQ211025005B
- Parameswaran P et al. 2020, "Phase evolution and characterization of mechanically alloyed hexanary Al16.6Mg16.6Ni16.6Cr16.6Ti16.6Mn16.6 high entropy alloy", Metal Powder Report, vol. 75, no. 4, doi:10.1016/j.mprp.2019.08.001
- Parish RV 1977, The Metallic Elements, Longman, London, ISBN:978-0-582-44278-8
- Partington JR 1944, A Text-book of Inorganic Chemistry, 5th ed., Macmillan & Co., London
- Petruševski VM & Cvetković J 2018, "On the 'true position' of hydrogen in the Periodic Table", Foundations of Chemistry, vol. 20, pp. 251–260, doi:10.1007/s10698-018-9306-y
- Phillips CSG & Williams RJP 1965, Inorganic Chemistry, vol. 1, Principles and non-metals, Clarendon Press, Oxford
- Phillips JC 1973, "The chemical structure of solids", in Hannay NB (ed.), Treatise on Solid State Chemistry, vol. 1, Plenum Press, New York, pp. 1–42, ISBN 978-1-4684-2663-2
- Piro NA et al. 2006, "Triple-bond reactivity of diphosphorus molecules", Science, vol. 313, no. 5791, doi:10.1126/science.1129630, PMID 16946068
- Pitzer K 1975, "Fluorides of radon and elements 118", Journal of the Chemical Society, Chemical Communications, no. 18, doi:10.1039/C3975000760B
- Porterfield WW 1993, Inorganic Chemistry, Academic Press, San Diego, ISBN 978-0-12-562980-5
- Povh B & Rosina M 2017, Scattering and Structures: Essentials and Analogies in Quantum Physics, 2nd ed., Springer, Berlin, doi:10.1007/978-3-662-54515-7
- Powell P & Timms P 1974, The Chemistry of the Non-Metals, Chapman and Hall, London, ISBN 978-0-412-12200-2
- Puddephatt RJ & Monaghan PK 1989, The Periodic Table of the Elements, 2nd ed., Clarendon Press, Oxford, ISBN:978-0-19-855516-2
- Rahm M, Zeng T & Hoffmann R 2019, "Electronegativity seen as the ground-state average valence electron binding energy", Journal of the American Chemical Society, vol. 141, no. 1, pp. 342−351, doi:10.1021/jacs.8b10246
- Rajarathnam GP & Assallo AM 2016, The Zinc/bromine Flow Battery: Materials Challenges and Practical Solutions for Technology Advancement, Springer, Singapore, p. 3, ISBN:978-981-287-645-4
- Rao KY 2002, Structural Chemistry of Glasses, Elsevier, Oxford, ISBN:978-0-08-043958-7
- Rao CNR & Ganguly PA 1986, "New criterion for the metallicity of elements", Solid State Communications, vol. 57, no. 1, pp. 5–6, doi:10.1016/0038-1098(86)90659-9
- Rayner-Canham G 2018, "Organizing the transition metals", in Scerri E & Restrepo G (Ed’s.) Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table, Oxford University, New York, ISBN:978-0-190-668532
- Rayner-Canham G 2020, The Periodic Table: Past, Present and Future, World Scientific, New Jersey, ISBN:978-981-121-850-7
- Regnault MV 1853, Elements of Chemistry, vol. 1, 2nd ed., Clark & Hesser, Philadelphia
- Reilly C 2002, Metal Contamination of Food, Blackwell Science, Oxford, ISBN:978-0-632-05927-0
- Remy H 1956, Treatise on Inorganic Chemistry, Anderson JS (trans.), Kleinberg J (ed.), vol. II, Elsevier, Amsterdam
- Renouf E 1901, "Lehrbuch der Anorganischen Chemie", Science, vol. 13, no. 320, doi:10.1126/science.13.320.268
- Restrepo G, Llanos EJ & Mesa H 2006, "Topological space of the chemical elements and its properties", Journal of Mathematical Chemistry, vol. 39, doi:10.1007/s10910-005-9041-1
- Ritter SK 2011, "The case of the missing xenon", Chemical & Engineering News, vol. 89, no. 9, ISSN 0009-2347
- Rochow EG 1966, The Metalloids, DC Heath and Company, Boston
- Rochow EG 1973, "Silicon", in Bailar JC et al. (eds.), Comprehensive Inorganic Chemistry, vol. 1, Pergamon Press, Oxford, ISBN 978-0-08-015655-2
- Rodgers GE 2012, Descriptive Inorganic, Coordination, and Solid State Chemistry, 3rd ed., Brooks/Cole, Belmont, California, ISBN 978-0-8400-6846-0
- Royal Society of Chemistry 2021, Periodic Table: Non-metal, accessed September 3, 2021
- Rudolph J 1973, Chemistry for the Modern Mind, Macmillan, New York
- Russell AM & Lee KL 2005, Structure-Property Relations in Nonferrous Metals, Wiley-Interscience, New York, ISBN:0-471-64952-X
- Salinas JT 2019 Exploring Physical Science in the Laboratory, Moreton Publishing, Englewood, Colorado, ISBN 978-1-61731-753-8
- Salzberg HW 1991, From Caveman to Chemist: Circumstances and Achievements, American Chemical Society, Washington, DC, ISBN:0-8412-1786-6
- Sanderson RT 1957, "An electronic distinction between metals and nonmetals", Journal of Chemical Education, vol. 34, no. 5, doi:10.1021/ed034p229
- Sanderson RT 1967, Inorganic Chemistry, Reinhold, New York
- Scerri E (ed.) 2013, 30-Second Elements: The 50 Most Significant Elements, Each Explained In Half a Minute, Ivy Press, London, ISBN 978-1-84831-616-4
- Schaefer JC 1968, "Boron" in Hampel CA (ed.), The Encyclopedia of the Chemical Elements, Reinhold, New York
- Schlager N & Lauer J (eds.) 2000, Science and Its Times: 1700–1799, volume 4 of Science and Its Times: Understanding the Social Significance of Scientific Discovery, Gale Group, ISBN:978-0-7876-3932-7
- Schmedt auf der Günne J, Mangstl M & Kraus F 2012, "Occurrence of difluorine F2 in nature—In situ proof and quantification by NMR spectroscopy", Angewandte Chemie International Edition, vol. 51, no. 31, doi:10.1002/anie.201203515
- Scott D 2014, Around the World in 18 Elements, Royal Society of Chemistry, e-book, ISBN:978-1-78262-509-4
- Scott EC & Kanda FA 1962, The Nature of Atoms and Molecules: A General Chemistry, Harper & Row, New York
- Seese WS & Daub GH 1985, Basic Chemistry, 4th ed., Prentice-Hall, Englewood Cliffs, NJ, ISBN 978-0-13-057811-2
- Segal BG 1989, Chemistry: Experiment and Theory, 2nd ed., John Wiley & Sons, New York, ISBN:0-471-84929-4
- Shanabrook BV, Lannin JS & Hisatsune IC 1981, "Inelastic light scattering in a onefold-coordinated amorphous semiconductor", Physical Review Letters, vol. 46, no. 2, 12 January, doi:10.1103/PhysRevLett.46.130
- Shang et al. 2021, "Ultrahard bulk amorphous carbon from collapsed fullerene", Nature, vol. 599, pp. 599–604, doi:10.1038/s41586-021-03882-9
- Sherwin E & Weston GJ 1966, Chemistry of the Non-metallic Elements, Pergamon Press, Oxford
- Shiell et al. 2021, "Bulk crystalline 4H-silicon through a metastable allotropic transition", Physical Review Letters, vol. 26, p 215701, doi:10.1103/PhysRevLett.126.215701
- Shkol’nikov EV 2010, "Thermodynamic characterization of the amphoterism of oxides M2O3 (M = As, Sb, Bi) and their hydrates in aqueous media, Russian Journal of Applied Chemistry, vol. 83, no. 12, pp. 2121–2127, doi:10.1134/S1070427210120104
- Sidorov TA 1960, "The connection between structural oxides and their tendency to glass formation", Glass and Ceramics, vol. 17, no. 11, doi:10.1007BF00670116
- Siekierski S & Burgess J 2002, Concise Chemistry of the Elements, Horwood Press, Chichester, ISBN:978-1-898563-71-6
- Smith A & Dwyer C 1991, Key Chemistry: Investigating Chemistry in the Contemporary World: Book 1: Materials and Everyday Life, Melbourne University Press, Carlton, Victoria, ISBN 978-0-522-84450-4
- Smits et al. 2020, "Oganesson: A noble gas element that is neither noble nor a gas", Angewandte Chemie International Edition, vol. 59, pp. 23636–23640, doi:10.1002/anie.202011976
- Stein L 1969, "Oxidized radon in halogen fluoride solutions", Journal of the American Chemical Society, vol. 19, no. 19, doi:10.1021/ja01047a042
- Stein L 1983, "The chemistry of radon", Radiochimica Acta, vol. 32, doi:10.1524/ract.1983.32.13.163
- Stellman JM (ed.) 1998, Encyclopaedia of Occupational Health and Safety, vol. 4, 4th ed., International Labour Office, Geneva, ISBN 978-92-2-109817-1
- Steudel R 1977, Chemistry of the Non-metals: With an Introduction to atomic Structure and Chemical Bonding, Walter de Gruyter, Berlin, ISBN:978-3-11-004882-7
- Steudel R & Eckert B 2003, "Solid sulfur allotropes", in Steudel R (ed.), Elemental Sulfur and Sulfur-rich Compounds I, Springer-Verlag, Berlin, ISBN:978-3-540-40191-9
- Steudel R 2020, Chemistry of the Non-metals: Syntheses - Structures - Bonding - Applications, in collaboration with D Scheschkewitz, Berlin, Walter de Gruyter, doi:10.1515/9783110578065
- Still B 2016 The Secret Life of the Periodic Table, Cassell, London, ISBN:978-1-84403-885-5
- Stott RWA 1956, Companion to Physical and Inorganic Chemistry, Longmans, Green and Co, London
- Stuke J 1974, "Optical and electrical properties of selenium", in Zingaro RA & Cooper WC (eds.), Selenium, Van Nostrand Reinhold, New York, pp. 174
- Strathern P 2000, Mendeleyev's dream: The Quest for the Elements, Hamish Hamilton, London, ISBN:978-0-8412-1786-7
- Su et al. 2020, "Advances in photonics of recently developed Xenes", Nanophotonics, vol. 9, no. 7, doi:10.1515/nanoph-2019-0561
- Suresh CH & Koga NA 2001, "A consistent approach toward atomic radii”, Journal of Physical Chemistry A, vol. 105, no. 24. doi:10.1021/jp010432b
- Tang et al. 2021, "Synthesis of paracrystalline diamond", Nature, vol. 599, pp. 605–610, doi:10.1038/s41586-021-04122-w
- Taniguchi M, Suga S, Seki M, Sakamoto H, Kanzaki H, Akahama Y, Endo S, Terada S & Narita S 1984, 'Core-exciton induced resonant photoemission in the covalent semiconductor black phosphorus', Solid State Communications, vo1. 49, no. 9, pp. 867–7, doi:10.1016/0038-1098(84)90441-1
- Taylor MD 1960, First Principles of Chemistry, Van Nostrand, Princeton
- The Chemical News and Journal of Physical Science 1864, "Notices of books: Manual of the Metalloids", vol. 9, p. 22
- The Chemical News and Journal of Physical Science 1897, "Notices of books: A Manual of Chemistry, Theoretical and Practical", by WA Tilden", vol. 75, pp. 188–189
- Thompson M 2004, Osmium tetroxide (OsO4), Molecule of the Month, (May), doi:10.6084/m9.figshare.5437084
- Thornton BF & Burdette SC 2010, "Finding eka-iodine: Discovery priority in modern times", Bulletin for the history of chemistry, vol. 35, no. 2, accessed September 14, 2021
- Tidy CM 1887, Handbook of Modern Chemistry, 2nd ed., Smith, Elder & Co., London
- Tregarthen L 2003, Preliminary Chemistry, Macmillan Education: Melbourne, ISBN 978-0-7329-9011-4
- Trenberth KE & Smith L 2005, "The mass of the atmosphere: A constraint on global analyses", Journal of Climate, vol. 18, no. 6, doi:10.1175/JCLI-3299.1
- Tshitoyan et al. 2019, "Unsupervised word embeddings capture latent knowledge from materials science literature", Nature, vol. 571, doi:10.1038/s41586-019-1335-8
- Tyler PM 1948, From the Ground Up: Facts and Figures of the Mineral Industries of the United States, McGraw-Hill, New York
- U.S. Geological Survey 1998, Mineral Commodity Summaries, U.S. Geological Survey, accessed 27 August 2023
- U.S. Geological Survey 2023, Mineral Commodity Summaries, U.S. Geological Survey
- Vassilakis AA, Kalemos A & Mavridis A 2014, "Accurate first principles calculations on chlorine fluoride ClF and its ions ClF±", Theoretical Chemistry Accounts, vol. 133, no. 1436, doi:10.1007/s00214-013-1436-7
- Vernon R 2013, "Which elements are metalloids?", Journal of Chemical Education, vol. 90, no. 12, 1703‒1707, doi:10.1021/ed3008457
- Vernon R 2020, "Organising the metals and nonmetals", Foundations of Chemistry, vol. 22, doi:10.1007/s10698-020-09356-6 (open access)
- Wächtershäuser G 2014, "From chemical invariance to genetic variability", in Weigand W and Schollhammer P (eds.), Bioinspired Catalysis: Metal Sulfur Complexes, Wiley-VCH, Weinheim, doi:10.1002/9783527664160.ch1
- Wakeman TH 1899, "Free thought—Past, present and future", Free Thought Magazine, vol. 17
- Wasewar KL 2021, "Intensifying approaches for removal of selenium", in Devi et al. (eds.), Selenium contamination in water, John Wiley & Sons, Hoboken, pp. 319–355, ISBN 978-1-119-69354-3
- Weeks ME 1945, Discovery of the Elements, 5th ed., Journal of Chemical Education, Easton, Pennsylvania
- Welcher SH 2001, High marks: Regents Chemistry Made Easy, 2nd ed., High Marks Made Easy, New York, ISBN:978-0-9714662-4-1
- Wells AF 1984, Structural Inorganic Chemistry, 5th ed., Clarendon Press, Oxford, ISBN:978-0-19-855370-0
- White JH 1962, Inorganic Chemistry: Advanced and Scholarship Levels, University of London Press, London
- Wibaut P 1951, Organic Chemistry, Elsevier Publishing Company, New York
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego, ISBN:978-0-12-352651-9
- Williams RPJ 2007, "Life, the environment and our ecosystem", Journal of Inorganic Biochemistry, vol. 101, nos. 11–12, doi:10.1016/j.jinorgbio.2007.07.006
- Woodward et al. 1999, "The electronic structure of metal oxides", In Fierro JLG (ed.), Metal Oxides: Chemistry and Applications, CRC Press, Boca Raton, ISBN:1-4200-2812-X
- Wulfsberg G 1987, Principles of Descriptive Chemistry, Brooks/Cole, Belmont CA, ISBN:978-0-534-07494-4
- Wulfsberg G 2000, Inorganic Chemistry, University Science Books, Sausalito, California, ISBN:978-1-891389-01-6
- Xia G-J, Ning Z-X & Zhu X-M 2020, “Effect of low-frequency oscillation on plasma focusing in krypton hall thruster", Journal of Propulsion and Power, vol. 36, no. 1, pp. Journal of Propulsion and Power, doi:10.2514/1.B37599
- Yamaguchi M & Shirai Y 1996, "Defect structures", in Stoloff NS & Sikka VK (eds.), Physical Metallurgy and Processing of Intermetallic Compounds, Chapman & Hall, New York, ISBN:978-1-4613-1215-4
- Yang J 2004, "Theory of thermal conductivity", in Tritt TM (ed.), Thermal Conductivity: Theory, Properties, and Applications, Kluwer Academic/Plenum Publishers, New York, pp. 1–20, ISBN 978-0-306-48327-1,
- Yoder CH, Suydam FH & Snavely FA 1975, Chemistry, 2nd ed, Harcourt Brace Jovanovich, New York, ISBN:978-0-15-506470-6
- Young JA 2006, "Iodine", Journal of Chemical Education, vol. 83, no. 9, doi:10.1021/ed083p1285
- Young et al. 2018, General Chemistry: Atoms First, Cengage Learning: Boston, ISBN 978-1-337-61229-6
- Yousuf M 1998, "Diamond anvil cells in high-pressure studies of semiconductors", in Suski T & Paul W (eds.), High Pressure in Semiconductor Physics II, Semiconductors and Semimetals, vol. 55, Academic Press, San Diego, ISBN:978-0-08-086453-2
- Zhao J, Tu Z & Chan SH 2021, "Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review", Journal of Power Sources, vol. 488, #229434, doi:10.1016/j.jpowsour.2020.229434
- Zhao Z, Zhang H, Kim D. et al. 2017, "Properties of the exotic metastable ST12 germanium allotrope", Nature Communications, vol. 8, article no. 13909, doi:10.1038/ncomms13909, PMID 28045027, PMC 5216117
- Zhigal'skii GP & Jones BK 2003, The Physical Properties of Thin Metal Films, Taylor & Francis, London, ISBN 978-0-415-28390-8
- Zhu W 2020, Chemical Elements In Life, World Scientific, Singapore, ISBN:978-981-121-032-7
- Zhu et al. 2014, "Reactions of xenon with iron and nickel are predicted in the Earth's inner core", Nature Chemistry, vol. 6, doi:10.1038/nchem.1925, PMID 24950336
- Zumdahl SS & DeCoste DJ 2010, Introductory Chemistry: A Foundation, 7th ed., Cengage Learning, Mason, Ohio, ISBN 978-1-111-29601-8
External links