Chemistry:Methylnaltrexone
 | |
| Clinical data | |
|---|---|
| Trade names | Relistor |
| Other names | MNTX, naltrexone-methyl-bromide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608052 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 11–15.3% |
| Metabolism | Liver |
| Elimination half-life | 8 hours |
| Excretion | Urine (50%), faeces (50%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
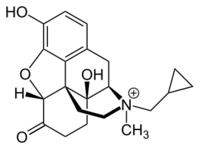
| Formula | C21H26NO4 |
| Molar mass | 356.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Methylnaltrexone (MNTX, brand name Relistor), used in form of methylnaltrexone bromide (INN, USAN, BAN), is a medication that acts as a peripherally acting μ-opioid receptor antagonist that acts to reverse some of the side effects of opioid drugs such as constipation without significantly affecting pain relief or precipitating withdrawals. Because MNTX is a quaternary ammonium cation, it cannot cross the blood–brain barrier, and so has antagonist effects throughout the body, counteracting effects such as itching and constipation, but without affecting opioid effects in the brain such as pain relief.[5] However, since a significant fraction (up to 60%) of opioid analgesia can be mediated by opioid receptors on peripheral sensory neurons, particularly in inflammatory conditions such as arthritis, traumatic or surgical pain,[6] MNTX may increase pain under such circumstances.
Medical uses
Methylnaltrexone is approved for the treatment of opioid-induced constipation in chronic non cancer pain or when ordinary laxatives have failed.[7]
Mechanism of action
Methylnaltrexone is a peripheral acting mu-opioid receptor antagonist, and does not cross the blood brain barrier.[8] Methylnaltrexone has restricted access through the blood brain barrier because it is a quaternary amine, which carries a positive charge when in a solution and more has polarity with lower lipid solubility than a lot of the opioid agonists used for pain treatment.[9] The peripheral action of methylnaltrexone makes it effective for decreasing the constipating effects of opioids, without interfering with the analgesic effects (of opioids) on the central nervous system.[9] This is the primary characteristic that makes methylnaltrexone behave differently than naltrexone.[10]
Furthermore, as methylnaltrexone cannot cross the blood–brain barrier, it does not reverse the pain-killing properties of opioid agonists or cause withdrawal symptoms, but since a small portion of analgesia comes from the peripheral opioid receptors, it can increase pain from inflammatory conditions such as arthritis.[citation needed]
Side effects
The most common side effects for methylnaltrexone include:[7][9]
- Abdominal pain
- Dizziness
- Vomiting
- Nausea
- Diarrhea
History
In 1978, a dying friend and colleague presented the late University of Chicago pharmacologist Leon Goldberg with a clinical challenge.[11] Struggling with the pain of prostatic cancer that had metastasized to his bones, the man was now declining the morphine he required for analgesia because of constipation. Research on opioids which would target only the sub-types of receptors associated with pain relief and not with side effects had seen little success outside of in-vitro models. Considering drugs such as loperamide, which acted on the opioid receptors in the gut without acting on the central nervous system, Goldberg proposed a targeted opioid receptor antagonist.[12]
Thousands of opioid-like molecules had been synthesized by pharmaceutical companies looking for the better analgesic - and many of those with no pain-relieving properties had been shelved. Screening these compounds led to the examination of putative antagonists which when modified had properties that suggested they might not readily cross the blood–brain barrier based on their size and charge. One of these compounds, N-methyl-naltrexone (MNTX), was amongst a group of compounds synthesized by Boehringer Ingelheim.[13] The compound looked promising and passed initial screening in which rodents were given opioids along with charcoal meals to track GI transit, and were tested for analgesia.[14] In a 1982 paper by Russell et al., it was first reported that the GI effects of the opioids could be prevented without affecting centrally mediated analgesia in this model.[15] Subsequent preclinical studies also demonstrated this separation of central and peripherally mediated opioid effects for other smooth muscles of the GI tract and the cough reflex.[16][17] Interest also developed in the potential for MNTX to act at the chemoreceptor trigger zone and block the emetic effect of opioids. This blockade of opioid-induced emesis was demonstrated in a canine model.[18][19] Goldberg died before he could see the core of this idea come into clinical practice.
Research on methylnaltrexone continued in the Department of Anesthesiology and Critical Care at the University of Chicago through the 1990s. More recent investigations, however, discovered opioid receptors on peripheral sensory neurons.[20]
In December 2005, Wyeth and Progenics entered into an exclusive, worldwide agreement for the joint development and commercialization of methylnaltrexone for the treatment of opioid-induced side effects, including constipation and post-operative ileus (POI), a prolonged dysfunction of the gastrointestinal tract following surgery. Under the terms of the agreement, the companies are collaborating on worldwide development. Wyeth received worldwide rights to commercialize methylnaltrexone, and Progenics retained an option to co-promote the product in the United States. Wyeth will pay Progenics royalties on worldwide sales and co-promotion fees within the United States.
Methylnaltrexone is being developed in subcutaneous and oral forms to treat opioid induced constipation (OIC).
The use of methylnaltrexone (Relistor) for more than 4 months has not been studied.[21]
Society and culture
Approval
On April 1, 2008, Progenics and Wyeth announced that Health Canada has approved methylnaltrexone for the treatment of opioid-induced constipation.[22] It was later approved by the US FDA on April 24, 2008.[23][24]
Forms
As of 2010, methylnaltrexone is supplied as an injection in trays containing seven one-dose vials containing 0.6 mL of solution. Each tray also contains seven 12 mm (0.47 in) 1 mL 27 gauge needles with retractable tips, and alcohol wipes for home use. A single vial can treat someone who weighs as much as 115 kilograms (254 lb).[10] For hospital use, vials are available separately.
See also
- Loperamide - an μ-opioid receptor agonist that doesn't cross the BBB in significant amounts, and treats diarrhea (in contrast to methynaltrexone, a Mu-opioid receptor antagonist that doesn't cross the BBB, avoiding opiate withdrawal effects in patients, while treating constipation)
- Naloxegol (trade names Movantik and Moventig) - another peripherally selective opioid antagonist used to treat opioid-induced constipation
- (+)-Naloxone - a non-opioid drug which also reduces some side effects of opioids without significantly affecting analgesia when used in small oral doses
- 6β-Naltrexol (6α-hydroxynaltrexone) - another naltrexone derivative that is also a peripherally selective opioid antagonist
References
- ↑ "Methylnaltrexone (Relistor) Use During Pregnancy". 9 July 2020. https://www.drugs.com/pregnancy/methylnaltrexone.html.
- ↑ "Relistor Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=79388.
- ↑ "Relistor Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=84164.
- ↑ "Relistor Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=84165.
- ↑ National Prescribing Service (1 March 2010). "Methylnaltrexone injections (Relistor) for opioid-induced constipation in palliative care". http://www.nps.org.au/health_professionals/publications/nps_radar/2010/march_2010/methylnaltrexone.
- ↑ "Peripheral mechanisms of opioid analgesia". Anesthesia and Analgesia 76 (1): 182–91. January 1993. doi:10.1213/00000539-199301000-00031. PMID 8380316.
- ↑ 7.0 7.1 "Highlights of Prescribing Information - Metylnaltrexone bromide". https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208271s000lbl.pdf.
- ↑ "Methylnaltrexone for opioid-induced constipation in advanced illness". The New England Journal of Medicine 358 (22): 2332–2343. May 2008. doi:10.1056/nejmoa0707377. PMID 18509120.
- ↑ 9.0 9.1 9.2 "Methylnaltrexone bromide: research update of pharmacokinetics following parenteral administration". Expert Opinion on Drug Metabolism & Toxicology 7 (2): 227–235. 11 January 2011. doi:10.1517/17425255.2011.549824. PMID 21222554.
- ↑ 10.0 10.1 "Relistor Full Prescribing Information". http://www.wyeth.com/content/showlabeling.asp?id=499.[yes|permanent dead link|dead link}}]
- ↑ "Drug developed at the University of Chicago wins FDA approval" (in en). 30 April 2008. https://news.uchicago.edu/story/drug-developed-university-chicago-wins-fda-approval.
- ↑ "Identifying and Treating Opioid Side Effects: The Development of Methylnaltrexone". Anesthesiology 130 (1): 142–148. January 2019. doi:10.1097/ALN.0000000000002428. PMID 30277930. https://anesthesiology.pubs.asahq.org/article.aspx?articleid=2705906.
- ↑ Karl Zeile and Kurt Freter & Freter, Kurt, "Quaternary salts of normorphine and its acylated derivatives", US patent 3101339, issued 1963-08-20, assigned to C. H. Boehringer Sohn. About Karl Zeile: born 1905. Untersuchungen über häminhaltige Fermente, habilitation treatise, Technische Hochschule, Munich 1933; In 1933 also SS-Member; 1937 NSDAP-Member; ao. (i.e. extra-ordinary) Professor in Göttingen; 1938 Delegate of NS-Dozentenbund; 1942 Prof. Organic Chemistry and biochemistry at Reichsuniversität Straßburg, and military research; after WW II deputy of Science-Dptm at Chemische Fabrik C. H. Boehringer Ingelheim
- ↑ Goldberg L, Merz H, Stockhaus K, "Quaternary derivatives of noroxymorphone which relieve intestinal immobility", US patent 4176186, issued 1979-11-27, assigned to Boehringer Ingelheim
- ↑ "Antagonism of gut, but not central effects of morphine with quaternary narcotic antagonists". European Journal of Pharmacology 78 (3): 255–61. March 1982. doi:10.1016/0014-2999(82)90026-7. PMID 7200037.
- ↑ "Effects of methylnaltrexone on morphine-induced inhibition of contraction in isolated guinea-pig ileum and human intestine". European Journal of Pharmacology 276 (1–2): 107–11. March 1995. doi:10.1016/0014-2999(95)00018-G. PMID 7781680.
- ↑ "Effects of methylnaltrexone on morphine-induced cough suppression in guinea pigs". Life Sciences 59 (15): PL235-8. 1996. doi:10.1016/0024-3205(96)00451-1. PMID 8845013.
- ↑ "Dose-related antagonism of the emetic effect of morphine by methylnaltrexone in dogs". Journal of Clinical Pharmacology 33 (8): 747–51. August 1993. doi:10.1002/j.1552-4604.1993.tb05618.x. PMID 8408737.
- ↑ Goldberg LI, "Quaternary derivatives of noroxymorphone which relieve nausea and emesis", US patent 4719215, issued 1988-01-12, assigned to University of Chicago
- ↑ "Attacking pain at its source: new perspectives on opioids". Nature Medicine 9 (8): 1003–8. August 2003. doi:10.1038/nm908. PMID 12894165.
- ↑ "Relistor Dosage and Administration". Wyeth. http://www.wyeth.com/hcp/relistor/information-14.
- ↑ "Wyeth press release - Wyeth and Progenics Announce Relistor Receives Canadian Marketing Approval". http://www.wyeth.com/news?nav=display&navTo=/wyeth_html/home/news/pressreleases/2008/1207054025120.html.
- ↑ "Wyeth press release - Progenics and Wyeth Announce FDA has Approved Relistor". http://www.wyeth.com/news?nav=display&navTo=/wyeth_html/home/news/pressreleases/2008/1209080590441.html.
- ↑ "FDA Approves Relistor for Opioid-Induced Constipation". https://www.fda.gov/bbs/topics/NEWS/2008/NEW01826.html.
Further reading
- "Treatment of opioid-induced gut dysfunction". Expert Opinion on Investigational Drugs 16 (2): 181–94. February 2007. doi:10.1517/13543784.16.2.181. PMID 17243938.
- "Oral methylnaltrexone for opioid-induced constipation". JAMA 284 (11): 1383–4. September 2000. doi:10.1001/jama.284.11.1383. PMID 10989399.
 |
