Chemistry:Tetrasulfur tetranitride
| |||
|
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
| IUPACName = Tetrasulfur tetranitride | SystematicName = 1,3,5,7-tetrathia-2,4,6,8-tetraazacyclooctan-2,4,6,8-tetrayl | Section1 = ! colspan=2 style="background: #f8eaba; text-align: center;" |Identifiers
|-
|
|
|-
|
|
|-
|
|-
|
|
|-
| UNII
|
|-
| colspan="2" |
- InChI=1S/N4S4/c1-5-2-7-4-8-3-6-1
 Key: LTPQFVPQTZSJGS-UHFFFAOYSA-N
Key: LTPQFVPQTZSJGS-UHFFFAOYSA-N
|-
| colspan="2" |
- N1=[S]N=[S]N=[S]N=[S]1
|- | Section2 = ! colspan=2 style="background: #f8eaba; text-align: center;" |Properties
|-
|
| S
4N
4
|- | Molar mass
| 184.287 g/mol
|- | Appearance | Vivid orange, opaque crystals |-
| Melting point
| 187 °C (369 °F; 460 K)
|-
| Section3 =
| Section4 =
| Section5 =
| Section6 =
}}
Tetrasulfur tetranitride is an inorganic compound with the formula S
4N
4. This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.[1][2]
Nitrogen and sulfur have similar electronegativities. When the properties of atoms are so highly similar, they often form extensive families of covalently bonded structures and compounds. Indeed, a large number of S-N and S-NH compounds are known with S
4N
4 as their parent.
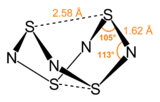
Structure
S
4N
4 adopts an unusual “extreme cradle” structure, with D2d point group symmetry. It can be viewed as a derivative of a (hypothetical) eight-membered ring (or more simply a 'deformed' eight-membered ring) of alternating sulfur and nitrogen atoms. The pairs of sulfur atoms across the ring are separated by 2.586 Å, resulting in a cage-like structure as determined by single crystal X-ray diffraction.[3] The nature of the transannular S–S interactions remains a matter of investigation because it is significantly shorter than the sum of the van der Waal's distances[4] but has been explained in the context of molecular orbital theory.[1] One pair of the transannular S atoms have valence 4, and the other pair of the transannular S atoms have valence 2.[citation needed] The bonding in S
4N
4 is considered to be delocalized, which is indicated by the fact that the bond distances between neighboring sulfur and nitrogen atoms are nearly identical. S
4N
4 has been shown to co-crystallize with benzene and the C
60 molecule.[5]
Properties
S
4N
4 is stable to air. It is, however, unstable in the thermodynamic sense with a positive enthalpy of formation of +460 kJ/mol. This endothermic enthalpy of formation originates in the difference in energy of S
4N
4 compared to its highly stable decomposition products:
- 2 S
4N
4 → 4 N
2 + S
8
Because one of its decomposition products is a gas, S
4N
4 can be used as an explosive.[1] Purer samples tend to be more explosive. Small samples can be detonated by striking with a hammer. S
4N
4 is thermochromic, changing from pale yellow below −30 °C to orange at room temperature to deep red above 100 °C.[1]
Synthesis
S
4N
4 was first prepared in 1835 by M. Gregory by the reaction of disulfur dichloride with ammonia,[6] a process that has been optimized:[7]
- 6 S
2Cl
2 + 16 NH
3 → S
4N
4 + S
8 + 12 [NH
4]Cl
Coproducts of this reaction include heptasulfur imide (S
7NH) and elemental sulfur. A related synthesis employs [NH
4]Cl instead:[1]
- 4 [NH
4]Cl + 6 S
2Cl
2 → S
4N
4 + 16 HCl + S
8
An alternative synthesis entails the use of (((CH
3)
3Si)
2N)
2S as a precursor with pre-formed S–N bonds. (((CH
3)
3Si)
2N)
2S is prepared by the reaction of lithium bis(trimethylsilyl)amide and SCl
2.
- 2 ((CH
3)
3Si)
2NLi + SCl
2 → (((CH
3)
3Si)
2N)
2S + 2 LiCl
The (((CH
3)
3Si)
2N)
2S reacts with the combination of SCl
2 and SO
2Cl
2 to form S
4N
4, trimethylsilyl chloride, and sulfur dioxide:[8]
- 2 (((CH
3)
3Si)
2N)
2S + 2 SCl
2 + 2 SO
2Cl
2 → S
4N
4 + 8 (CH
3)
3SiCl + 2 SO
2
Acid-base reactions
S
4N
4 serves as a Lewis base by binding through nitrogen to strongly Lewis acidic compounds such as SbCl
5 and SO
3. The cage is distorted in these adducts.[1]
- S
4N
4 + SbCl
5 → S
4N
4 · SbCl
5 - S
4N
4 + SO
3 → S
4N
4 · SO
3
The reaction of [Pt
2Cl
4(P(CH
3)
2Ph)
2] with S
4N
4 is reported to form a complex where a sulfur forms a dative bond to the metal. This compound upon standing is isomerised to a complex in which a nitrogen atom forms the additional bond to the metal centre.
It is protonated by H[BF
4] to form a tetrafluoroborate salt:
- S
4N
4 + H[BF
4] → [S
4N
4H]+
[BF
4]−
The soft Lewis acid CuCl forms a coordination polymer:[1]
- n S
4N
4 + n CuCl → (S
4N
4)
n-μ-(–Cu–Cl–)
n
Dilute NaOH hydrolyzes S
4N
4 as follows, yielding thiosulfate and trithionate:[1]
- 2 S
4N
4 + 6 OH−
+ 9 H
2O → S
2O2−
3 + 2 S
3O2−
6 + 8 NH
3
More concentrated base yields sulfite:
- S
4N
4 + 6 OH−
+ 3 H
2O → S
2O2−
3 + 2 SO2−
3 + 4 NH
3
Metal complexes
S
4N
4 reacts with metal complexes. The cage remains intact in some cases but in other cases, it is degraded.[2][9] S
4N
4 reacts with Vaska's complex ([Ir(Cl)(CO)(PPh
3)
2] in an oxidative addition reaction to form a six coordinate iridium complex where the S
4N
4 binds through two sulfur atoms and one nitrogen atom.
S
4N
4 as a precursor to other S-N compounds
Many S-N compounds are prepared from S
4N
4.[10] Reaction with piperidine generates [S
4N
5]−
:
- 24 S
4N
4 + 32 C
5H
10NH → 8 [C
5H
10NH
2]+
[S
4N
5]−
+ 8 (C
5H
10N)
2S + 3 S
8 + 8 N
2
A related cation is also known, i.e. [S
4N
5]+
.
Treatment with tetramethylammonium azide produces the heterocycle [S
3N
3]−
:
- 8 S
4N
4 + 8 [(CH
3)
4N]+
[N
3]−
→ 8 [(CH
3)
4N]+
[S
3N
3]−
+ S
8 + 16 N
2
Cyclo-[S
3N
3]−
has 10 pi-electrons.
In a related reaction, the use of the bis(triphenylphosphine)iminium azide gives a salt containing the blue [NS
4]−
anion:[10]
- 4 S
4N
4 + 2 [PPN]+
[N
3]−
→ 2 [PPN]+
[NS
4]−
+ S
8 + 10 N
2
The anion [NS
4]−
has a chain structure described using the resonance [S=S=N–S–S−
] ↔ [−
S–S–N=S=S].
S
4N
4 reacts with electron-poor alkynes.[11]
Chlorination of S
4N
4 gives thiazyl chloride.
Passing gaseous S
4N
4 over silver metal yields the low temperature superconductor polythiazyl or polysulfurnitride (transition temperature (0.26±0.03) K[12]), often simply called "(SN)x". In the conversion, the silver first becomes sulfided, and the resulting Ag
2S catalyzes the conversion of the S
4N
4 into the four-membered ring S
2N
2, which readily polymerizes.[1]
- S
4N
4 + 8 Ag → 4 Ag
2S + 2 N
2 - x S
4N
4 → (SN)
4x
Related compounds
- The selenium analogue Se
4N
4, tetraselenium tetranitride.
Safety
S
4N
4 is shock-sensitive. Purer samples are more shock-sensitive than those contaminated with elemental sulfur.[7]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Greenwood, N. N.; Earnshaw, A. (1997). Chemical Elements (2nd ed.). Boston, MA: Butterworth-Heinemann. pp. 721–725.
- ↑ Jump up to: 2.0 2.1 Chivers, T. (2004). A Guide To Chalcogen-Nitrogen Chemistry. Singapore: World Scientific Publishing. ISBN 981-256-095-5.
- ↑ Sharma, B. D.; Donohue, J. (1963). "The Crystal and Molecular Structure of Sulfur Nitride, S4N4". Acta Crystallographica 16 (9): 891–897. doi:10.1107/S0365110X63002401.
- ↑ Rzepa, H. S.; Woollins, J. D. (1990). "A PM3 SCF-MO Study of the Structure and Bonding in the Cage Systems S4N4 and S4N4X (X = N+, N−, S, N2S, P+, C, Si, B− and Al−)". Polyhedron 9 (1): 107–111. doi:10.1016/S0277-5387(00)84253-9.
- ↑ Konarev, D. V.; Lyubovskaya, R. N.; Drichko, N. V. et al. (2000). "Donor-Acceptor Complexes of Fullerene C60 with Organic and Organometallic Donors". Journal of Materials Chemistry 10 (4): 803–818. doi:10.1039/a907106g.
- ↑ Jolly, W. L.; Lipp, S. A. (1971). "Reaction of Tetrasulfur Tetranitride with Sulfuric Acid". Inorganic Chemistry 10 (1): 33–38. doi:10.1021/ic50095a008. https://escholarship.org/uc/item/7xj1q0zf.
- ↑ Jump up to: 7.0 7.1 Villena-Blanco, M. et al. (1967). S. Y. Tyree Jr. ed. "Tetrasulfur Tetranitride, S4N4". Inorganic Syntheses 9: 98–102. doi:10.1002/9780470132401.ch26.
- ↑ Maaninen, A.; Shvari, J.; Laitinen, R. S.; Chivers, T (2002). Coucouvanis, Dimitri. ed. "Compounds of General Interest". Inorganic Syntheses 33: 196–199. doi:10.1002/0471224502.ch4. ISBN 9780471208259.
- ↑ Kelly, P. F.; Slawin, A. M. Z.; Williams, D. J.; Woollins, J. D. (1992). "Caged explosives: Metal-Stabilized Chalcogen Nitrides". Chemical Society Reviews 21 (4): 245–252. doi:10.1039/CS9922100245.
- ↑ Jump up to: 10.0 10.1 Bojes, J. et al. (1989). Allcock, H. R.. ed. "Binary Cyclic Nitrogen-Sulfur Anions". Inorganic Syntheses 25: 30–35. doi:10.1002/9780470132562.ch7. ISBN 9780470132562.
- ↑ Dunn, P. J.; Rzepa, H. S. (1987). "The Reaction Between Tetrasulphur Tetranitride (S4N4) and Electron-deficient Alkynes. A Molecular Orbital Study". Journal of the Chemical Society, Perkin Transactions 2 1987 (11): 1669–1670. doi:10.1039/p29870001669.
- ↑ Greene, R. L.; Street, G. B.; Suter, L. J. (1975). "Superconductivity in Polysulfur Nitride (SN)x". Physical Review Letters 34 (10): 577–579. doi:10.1103/PhysRevLett.34.577. Bibcode: 1975PhRvL..34..577G.
 |