Chemistry:Mirvetuximab soravtansine
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric |
| Target | folate receptor alpha |
| Clinical data | |
| Trade names | Elahere |
| Other names | mirvetuximab soravtansine-gynx |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| UNII | |
| KEGG | |
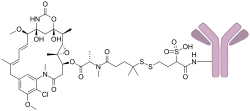
Mirvetuximab soravtansine, sold under the brand name Elahere, is a medication used as a treatment for fallopian tube cancer or primary peritoneal cancer.[1][2] Mirvetuximab soravtansine is a folate receptor alpha directed antibody and microtubule inhibitor conjugate.[2]
The most common adverse reactions, including laboratory abnormalities, were vision impairment, fatigue, increased aspartate aminotransferase, nausea, increased alanine aminotransferase, keratopathy, abdominal pain, decreased lymphocytes, peripheral neuropathy, diarrhea, decreased albumin, constipation, increased alkaline phosphatase, dry eye, decreased magnesium, decreased leukocytes, decreased neutrophils, and decreased hemoglobin.[2]
Mirvetuximab soravtansine was approved for medical use in the United States in November 2022.[2][3] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[4][5]
Medical uses
Mirvetuximab soravtansine is indicated for the treatment of adults with folate receptor alpha (FRα) positive, platinum-resistant epithelial ovarian,[6][7] fallopian tube, or primary peritoneal cancer, who have received one to three prior systemic treatment regimens.[1][2] Recipients are selected for therapy based on an FDA-approved test.[2]
Adverse effects
The product labeling includes a boxed warning for ocular toxicity.[1][2]
History
Efficacy was evaluated in Study 0417 (NCT04296890), a single-arm trial of 106 participants with FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.[2] Participants were permitted to receive up to three prior lines of systemic therapy.[2] All participants were required to have received bevacizumab.[2] The trial enrolled participants whose tumors were positive for FRα expression as determined by the above assay.[2] Participants were excluded if they had corneal disorders, ocular conditions requiring ongoing treatment, Grade >1 peripheral neuropathy, or noninfectious interstitial lung disease.[2]
Society and culture
Names
Mirvetuximab soravtansine is the international nonproprietary name (INN).[8]
References
- ↑ 1.0 1.1 1.2 1.3 "Elahere- mirvetuximab soravtansine injection, solution". 18 November 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=00c424b5-6ccd-48ab-9e88-1986451120e2.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer". 14 November 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Mirvetuximab Soravtansine: First Approval". Drugs 83 (3): 265–273. February 2023. doi:10.1007/s40265-023-01834-3. PMID 36656533. https://figshare.com/articles/online_resource/Mirvetuximab_Soravtansine_First_Approval/21788783.
- ↑ "Advancing Health Through Innovation: New Drug Therapy Approvals 2022". 10 January 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ (PDF) New Drug Therapy Approvals 2022 (Report). January 2024. https://www.fda.gov/media/164429/download. Retrieved 14 January 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Mirvetuximab soravtansine for platinum-resistant epithelial ovarian cancer". Expert Review of Anticancer Therapy 23 (8): 783–796. 2023. doi:10.1080/14737140.2023.2236793. PMID 37458180.
- ↑ "Mirvetuximab Soravtansine in Platinum-Resistant Ovarian Cancer". Clinical Medicine Insights. Oncology 17: 11795549231187264. 2023. doi:10.1177/11795549231187264. PMID 37528890.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 75". WHO Drug Information 30 (1). 2016.
External links
- "Mirvetuximab soravtansine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/mirvetuximab%20soravtansine.
- Clinical trial number NCT04296890 for "A Study of Mirvetuximab Soravtansine in Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression (SORAYA)" at ClinicalTrials.gov
 |

