Chemistry:5α-Dihydrocortisol
From HandWiki
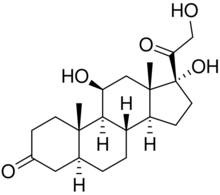
| |
| Names | |
|---|---|
| IUPAC name
11β,17α,21-Trihydroxy-5α-pregnane-3,20-dione
| |
| Systematic IUPAC name
(1R,3aS,3bS,5aS,9aS,9bS,10S,11aS)-1,10-Dihydroxy-1-(hydroxyacetyl)-9a,11a-dimethylhexadecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
Hydrallostane; 5α-DHF; Allodihydrocortisol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H32O5 | |
| Molar mass | 364.482 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5α-Dihydrocortisol (5α-DHF), also known as hydrallostane or as allodihydrocortisol,[1] is a metabolite of cortisol that is formed by 5α-reductase.[2][3] It is present in the aqueous humor of the eye, is produced in the lens of the eye, and is involved in regulating the formation of the aqueous humor.[2] 5α-DHF can be further metabolized into 3α,5α-tetrahydrocortisol by 3α-hydroxysteroid dehydrogenase.[3]
References
- ↑ "Hydrallostane". https://pubchem.ncbi.nlm.nih.gov/compound/12816693.
- ↑ 2.0 2.1 "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Adv Urol 2012: 530121. 2012. doi:10.1155/2012/530121. PMID 22235201.
- ↑ 3.0 3.1 "The role of 5alpha-reduction in steroid hormone physiology". Reprod. Fertil. Dev. 13 (7–8): 673–8. 2001. doi:10.1071/RD01074. PMID 11999320.
 |
