Chemistry:16β,17α-Epiestriol
From HandWiki
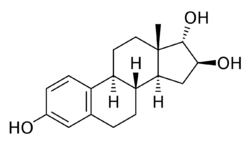
| |
| Names | |
|---|---|
| IUPAC name
Estra-1,3,5(10)-triene-3,16β,17α-triol
| |
| Systematic IUPAC name
(1S,2S,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,2,7-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H24O3 | |
| Molar mass | 288.387 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
16β,17α-Epiestriol, or 16,17-epiestriol, also known as 16β-hydroxy-17α-estradiol, as well as estra-1,3,5(10)-triene-3,16β,17α-triol, is a minor and weak endogenous steroidal estrogen that is related to 17α-estradiol and estriol.[1][2] Along with estriol, 16β,17α-epiestriol has been detected in the urine of women during the late pregnancy stage.[2] It shows preferential affinity for the ERβ over the ERα.[3]
See also
- 16β-Epiestriol
- 17α-Epiestriol
- Epimestrol
References
- ↑ Vitamins and Hormones. Academic Press. 18 April 1972. pp. 233–. ISBN 978-0-08-086626-0. https://books.google.com/books?id=iQZgQRUI_CgC&pg=PA233.
- ↑ 2.0 2.1 Ryō Satō; Ryūichi Katō (1982). Microsomes, drug oxidations, and drug toxicity. Japan Scientific Societies Press. p. 273. ISBN 978-0-471-87285-6. https://books.google.com/books?id=t7PwAAAAMAAJ.
- ↑ "Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding". Endocrinology 147 (9): 4132–50. 2006. doi:10.1210/en.2006-0113. PMID 16728493.
 |
