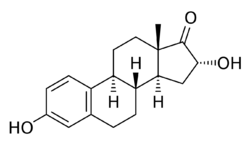
Chemistry:16α-Hydroxyestrone
| |
| Names | |
|---|---|
| IUPAC name
3,16α-Dihydroxyestra-1,3,5(10)-trien-17-one
| |
| Systematic IUPAC name
(2R,3aS,3bR,9bS,11aS)-2,7-Dihydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-1-one | |
| Other names
Hydroxyestrone; 16-Hydroxyestrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H22O3 | |
| Molar mass | 286.371 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-triene-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol.[1][2] It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer.[1] Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.[1]
In terms of relative binding affinity (RBA) for the rat uterine estrogen receptor, 16α-hydroxyestrone showed 2.8% of the affinity of estradiol.[3] For comparison, estrone had 11% of the affinity and estriol had 10% of the affinity of estradiol.[3] In contrast to other estrogens, the binding of 16α-hydroxyestrone to the estrogen receptor is reported to be covalent and irreversible.[4][5][6][7] 16α-Hydroxyestrone has been reported to have 25% of the vaginal estrogenic potency of estradiol.[3] The maximal uterotrophic and antigonadotropic effect of 16α-hydroxyestrone was equivalent to those of estradiol and estriol, indicating that 16α-hydroxyestrone is a fully effective estrogen.[3][8] However, 16α-hydroxyestrone was much less potent than estradiol or estrone.[8]
The C3 and C16α diacetate ester of 16α-hydroxyestrone, hydroxyestrone diacetate (brand names Colpoginon, Colpormon, Hormobion, and Hormocervix), has been marketed and used medically as an estrogen in Europe.[9][10]
See also
- 2-Hydroxyestrone
- 2-Hydroxyestradiol
- 16β-Hydroxyestrone
- 16-Ketoestrone
- 16α-Hydroxydehydroepiandrosterone
- 16α-Hydroxyandrostenedione
- 15α-Hydroxydehydroepiandrosterone
References
- ↑ 1.0 1.1 1.2 Rakel, David (2012). Integrative Medicine. Elsevier Health Sciences. pp. 338–339. ISBN 978-1-4377-1793-8. https://books.google.com/books?id=jlVtJzBwAcEC&pg=PA338.
- ↑ Vitamins and Hormones. Academic Press. 7 September 2005. pp. 282–. ISBN 978-0-08-045978-3. https://books.google.com/books?id=DUst5mwBfN0C&pg=PA282.
- ↑ 3.0 3.1 3.2 3.3 "Biological properties of 16 alpha-hydroxyestrone: implications in estrogen physiology and pathophysiology". J. Clin. Endocrinol. Metab. 51 (3): 611–5. September 1980. doi:10.1210/jcem-51-3-611. PMID 7190977.
- ↑ Oettel, Michael; Schillinger, Ekkehard (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 252–. ISBN 978-3-642-58616-3. https://books.google.com/books?id=0BfrCAAAQBAJ&pg=PA252.
- ↑ "Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization". Proc. Natl. Acad. Sci. U.S.A. 85 (21): 7831–5. November 1988. doi:10.1073/pnas.85.21.7831. PMID 3186693. Bibcode: 1988PNAS...85.7831S.
- ↑ "Functional role of estrogen metabolism in target cells: review and perspectives". Carcinogenesis 19 (1): 1–27. January 1998. doi:10.1093/carcin/19.1.1. PMID 9472688.
- ↑ "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf.
- ↑ 8.0 8.1 Velardo, Joseph Thomas (1964). "The Actions of Steroid Hormones on Estradiol-17β in Uterine Growth and Enzymorphology". Hormonal Steroids Biochemistry, Pharmacology, and Therapeutics. pp. 463–490. doi:10.1016/B978-0-12-395506-7.50065-0. ISBN 9780123955067.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1250–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA1250.
- ↑ Muller, Niels F; Dessing, Rudolf P; European Society of Clinical Pharmacy (19 June 1998). European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. pp. 289–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA289.
 |
