Chemistry:17α-Epiestriol
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
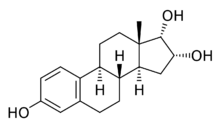
Estra-1,3,5(10)-triene-3,16α,17α-triol
| |
| Systematic IUPAC name
(1S,2R,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,2,7-triol | |
| Other names
17-Epiestriol; 16α-Hydroxy-17α-estradiol; 3,16α,17α-Trihydroxy-1,3,5(10)-estratriene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H24O3 | |
| Molar mass | 288.38136 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol (which is 16α-hydroxy-17β-estradiol).[1][2][3] It is formed from 16α-hydroxyestrone.[4][5] In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ.[6] It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα.[7] 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression in vitro.[8]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | ||
|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7 | <0.1 | ||
| Alfatradiol | <1 | <1 | 15 | <1 | <1 | ? | ? | ||
| Estriol | <1 | <1 | 15 | <1 | <1 | ? | ? | ||
| 16β-Epiestriol | <1 | <1 | 20 | <1 | <1 | ? | ? | ||
| 17α-Epiestriol | <1 | <1 | 31 | <1 | <1 | ? | ? | ||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. | |||||||||
See also
- Epimestrol
- 16β,17α-Epiestriol
- 16β-Epiestriol
- 17α-Estradiol
- 2-Methoxyestradiol
References
- ↑ Tewari, Ashutosh K (5 April 2013). Prostate Cancer: A Comprehensive Perspective. Springer Science & Business Media. pp. 373–. ISBN 978-1-4471-2864-9. https://books.google.com/books?id=8VI_AAAAQBAJ&pg=PA373.
- ↑ Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. 6 December 2012. pp. 522–. ISBN 978-3-642-96158-8. https://books.google.com/books?id=DAgJCAAAQBAJ&pg=PA522.
- ↑ The Maternal Organism. Elsevier. 3 September 2013. pp. 341–. ISBN 978-1-4832-6380-9. https://books.google.com/books?id=mSzLBAAAQBAJ&pg=PA341.
- ↑ Comparative Endocrinology. Elsevier Science. 2 December 2012. pp. 135–. ISBN 978-0-323-14609-8. https://books.google.com/books?id=rAJX4z_oQrkC&pg=PA135.
- ↑ Tietz, Norbert W. (1 August 1976). Fundamentals of clinical chemistry. Saunders. p. 773. ISBN 978-0-7216-8866-4. https://books.google.com/books?id=Keg6AAAAMAAJ.
- ↑ Sherbet, Gajanan V. (26 July 2013). Therapeutic Strategies in Cancer Biology and Pathology. Elsevier. pp. 83–. ISBN 978-0-12-416590-8. https://books.google.com/books?id=vSSES5WRdZ4C&pg=PA83.
- ↑ Dorfman, Ralph I. (22 October 2013). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 13–. ISBN 978-1-4832-7299-3. https://books.google.com/books?id=BbLfBAAAQBAJ&pg=PA13.
- ↑ "17-epiestriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (VCAM-1) mRNA expression". The Journal of Biological Chemistry 278 (14): 11746–52. April 2003. doi:10.1074/jbc.M207800200. PMID 12547825.
- ↑ Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids". Drug Design. pp. 169–214. doi:10.1016/B978-0-12-060308-4.50010-X. ISBN 9780120603084. https://books.google.com/books?id=bhAlBQAAQBAJ&pg=PA169.
- ↑ "Unique steroid congeners for receptor studies". Cancer Research 38 (11 Pt 2): 4186–98. November 1978. PMID 359134. http://cancerres.aacrjournals.org/content/38/11_Part_2/4186.short.
- ↑ "Towards the mapping of the progesterone and androgen receptors". Journal of Steroid Biochemistry 27 (1–3): 255–69. 1987. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ↑ "Steroid hormone receptors and pharmacology". Journal of Steroid Biochemistry 12: 143–57. January 1980. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
 |
