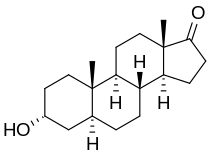
Biology:Androsterone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3α-hydroxy-5α-androstan-17-one, 5α-androstan-3α-ol-17-one |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone.[1] It is a weak androgen with a potency that is approximately 1/7 that of testosterone.[2] Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.[3][4]
Androsterone is also known to be an inhibitory androstane neurosteroid,[5][6] acting as a positive allosteric modulator of the GABAA receptor,[7] and possesses anticonvulsant effects.[8] The unnatural enantiomer of androsterone is more potent as a positive allosteric modulator of GABAA receptors and as an anticonvulsant than the natural form.[9] Androsterone's 3β-isomer is epiandrosterone, and its 5β-epimer is etiocholanolone. The 3β,5β-isomer is epietiocholanolone.
Biological function
Androsterone has generally been considered to be an inactive metabolite of testosterone, which when conjugated by glucuronidation and sulfation allows testosterone to be removed from the body, but it is a weak neurosteroid that can cross into the brain and could have effects on brain function.[8]
The view of androsterone as generally being of low significance however, seems to need review in the light of 21st century research, which suggests that androsterone significantly affects masculinization in mammalian fetuses. Masculinization of the external genitalia in humans is subject to dihydrotestosterone (DHT) derived via the recognised androgenic pathway and also via a backdoor pathway.[10] Therefore, androstanediol can be used a marker of the backdoor pathway of DHT synthesis.[11] Spectrometric studies identify androsterone as the main backdoor androgen in the human male fetus. Circulating levels are sex dependent, DHT being essentially absent in the female, in which titres of backdoor intermediates also are very low.[12]
In males, backdoor intermediates occur mainly in the liver and adrenal of the fetus, and in the placenta — hardly at all in the testis. Instead, progesterone in the placenta is the main backdoor substrate for androgen synthesis. This also is consistent with the observation that placental insufficiency has been associated with disruptions of development of fetal genitalia.[12]
Pheromone
Androsterone is found in the human axilla and skin as well as in the urine.[13] It may also be secreted by human sebaceous glands.[13] It is described as having a musky odor similar to that of androstenol.[13] Androsterone has been found to affect human behavior when smelled.[13]
Biochemistry
Biosynthesis
Androsterone and its 5β-isomer, etiocholanolone, are produced in the body as metabolites of testosterone. Testosterone is converted to 5α-dihydrotestosterone and 5β-dihydrotestosterone by 5α-reductase and 5β-reductase, respectively. The enzyme 3α-hydroxysteroid dehydrogenase converts the reduced forms to 3α-androstanediol and 3β-androstanediol, which are subsequently converted by 17β-hydroxysteroid dehydrogenase to androsterone and etiocholanolone, respectively. Androsterone and etiocholanolone can also be formed from androstenedione via the action of 5α-reductase and 5β-reductase forming 5α-androstanedione and 5β-androstanedione which are then converted to androsterone and etiocholanolone by 3α-hydroxysteroid dehydrogenase and 3β-hydroxysteroid dehydrogenase, respectively.[8]
Metabolism
Androsterone is sulfated into androsterone sulfate and glucuronidated into androsterone glucuronide and these conjugates are excreted in urine.
Chemistry
Sources
Androsterone has been shown to naturally occur in pine pollen, celery, truffles and is well known in many animal species.[14][15]
History
Androsterone was first isolated in 1931, by Adolf Friedrich Johann Butenandt and Kurt Tscherning. They distilled over 17,000 liters (3,700 imp gal; 4,500 U.S. gal) of male urine, from which they got 50 milligrams (0.77 gr) of crystalline androsterone, which was sufficient to find that the chemical formula was very similar to estrone.
See also
- Androgen backdoor pathway
- List of androgens/anabolic steroids
- List of neurosteroids § Androstanes
- List of neurosteroids § Pheromones and pherines
References
- ↑ "A dual physiological character for cerebral mechanisms of sexuality and cognition: common somatic peripheral afferents". BJU International 108 (10): 1634–1639. November 2011. doi:10.1111/j.1464-410X.2011.10116.x. PMID 21489118.
- ↑ Concise Encyclopedia Biology. Walter de Gruyter. 1996. p. 49. ISBN 978-3-11-010661-9. https://books.google.com/books?id=LorrYj5pkKYC&pg=PA49. Retrieved May 25, 2012.
- ↑ "Biosynthesis, Transport, and Metabolism of Steroid HormonesHenderson BE, Ponder BA, Ross RK". Hormones, Genes, and Cancer. Oxford University Press. March 13, 2003. p. 23. ISBN 978-0-19-513576-3. https://books.google.com/books?id=VzIWd4faVVQC&pg=PA23. Retrieved May 25, 2012.
- ↑ "Increased activation of the alternative "backdoor" pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis". The Journal of Clinical Endocrinology and Metabolism 97 (3): E367–E375. March 2012. doi:10.1210/jc.2011-1997. PMID 22170725.
- ↑ "Neurosteroids — Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy". Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US). National Center for Biotechnology Information (US). 2012. https://www.ncbi.nlm.nih.gov/books/NBK98218/.
- ↑ "Neurosteroids". Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research. 186. 2010. pp. 113–137. doi:10.1016/B978-0-444-53630-3.00008-7. ISBN 9780444536303.
- ↑ "Natural and enantiomeric etiocholanolone interact with distinct sites on the rat alpha1beta2gamma2L GABAA receptor". Molecular Pharmacology 71 (6): 1582–1590. June 2007. doi:10.1124/mol.106.033407. PMID 17341652.
- ↑ 8.0 8.1 8.2 "Anticonvulsant activity of androsterone and etiocholanolone". Epilepsia 46 (6): 819–827. June 2005. doi:10.1111/j.1528-1167.2005.00705.x. PMID 15946323.
- ↑ "Anticonvulsant potencies of the enantiomers of the neurosteroids androsterone and etiocholanolone exceed those of the natural forms". Psychopharmacology 231 (17): 3325–3332. September 2014. doi:10.1007/s00213-014-3546-x. PMID 24705905.
- ↑ "Alternative androgen pathways". WikiJournal of Medicine 10: X. 2023. doi:10.15347/WJM/2023.003.
- ↑ "The role of gonadotropins in testicular and adrenal androgen biosynthesis pathways-Insights from males with congenital hypogonadotropic hypogonadism on hCG/rFSH and on testosterone replacement". Clinical Endocrinology 94 (1): 90–101. January 2021. doi:10.1111/cen.14324. PMID 32871622.
- ↑ 12.0 12.1 "Alternative (backdoor) androgen production and masculinization in the human fetus". PLOS Biology 17 (2): e3000002. February 2019. doi:10.1371/journal.pbio.3000002. PMID 30763313.
- ↑ 13.0 13.1 13.2 13.3 "Influence of Androstenol and Androsterone on the Evalulation of Men of Varying Attractiveness Levels". Chemical Signals in Vertebrates 6. 1992. pp. 575–579. doi:10.1007/978-1-4757-9655-1_88. ISBN 978-1-4757-9657-5.
- ↑ "Mammalian sex hormones in plants". Folia Histochemica et Cytobiologica 43 (2): 71–79. 2005. PMID 16044944.
- ↑ "Ability to perceive androstenone can be acquired by ostensibly anosmic people". Proceedings of the National Academy of Sciences of the United States of America 86 (20): 7976–7978. October 1989. doi:10.1073/pnas.86.20.7976. PMID 2813372. Bibcode: 1989PNAS...86.7976W.
External links
 |
