Chemistry:Estriol glucuronide
 | |
| Clinical data | |
|---|---|
| Other names | Estriol glucuronidate; (16α,17β)-16,17-Dihydroxyestra-1,3,5(10)-trien-3-yl D-glucopyranosiduronic acid; β-D-Glucopyranuronic acid, monoglycoside with (16α,17β)-estra-1,3,5(10)-triene-3,16,17-triol |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C24H32O9 |
| Molar mass | 464.511 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
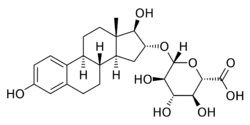
Estriol glucuronide (E3G), or oestriol glucuronide, also known as estriol monoglucuronide, as well as estriol 16α-β-D-glucosiduronic acid, is a natural, steroidal estrogen and the glucuronic acid (β-D-glucopyranuronic acid) conjugate of estriol.[1][2] It occurs in high concentrations in the urine of pregnant women as a reversibly formed metabolite of estriol.[2] Estriol glucuronide is a prodrug of estriol,[3] and was the major component of Progynon and Emmenin, estrogenic products manufactured from the urine of pregnant women that were introduced in the 1920s and 1930s and were the first orally active estrogens.[4][5] Emmenin was succeeded by Premarin (conjugated equine estrogens), which is sourced from the urine of pregnant mares and was introduced in 1941.[4][5][6] Premarin replaced Emmenin due to the fact that it was easier and less expensive to produce.[4][5]
Estrogen glucuronides can be deglucuronidated into the corresponding free estrogens by β-glucuronidase in tissues that express this enzyme, such as the mammary gland.[7] As a result, estrogen glucuronides have estrogenic activity via conversion into estrogens.[7]
The positional isomer of estriol 16α-glucuronide, estriol 3-glucuronide, also occurs as an endogenous metabolite of estriol, although to a much lower extent in comparison.[8][9][10]
See also
- Catechol estrogen
- Estradiol glucuronide
- Estradiol sulfate
- Estrogen conjugate
- Estrone glucuronide
- Estrone sulfate
- Lipoidal estradiol
References
- ↑ Dictionary of Steroids. CRC Press. 23 May 1991. pp. 274–. ISBN 978-0-412-27060-4. https://books.google.com/books?id=AI7EnUyeEtUC&pg=PA274.
- ↑ 2.0 2.1 "Isolation and characterization of estriol 16 alpha-glucosiduronic acid from human pregnancy urine". The Journal of Biological Chemistry 238 (4): 1273–1282. April 1963. doi:10.1016/S0021-9258(18)81175-9. PMID 14010351.
- ↑ "Implication of the Conjugation of Drug and Other Exogenous Substances". Glucuronic Acid Free and Combined: Chemistry, Biochemistry, Pharmacology, and Medicine. Elsevier. 2 December 2012. pp. 457–492 (466). ISBN 978-0-323-14398-1. https://books.google.com/books?id=wo5M1g9pjcMC&pg=PA466.
- ↑ 4.0 4.1 4.2 "Chapter 3: Life after the Thyroid". The Quest for Cortisone. MSU Press. 1 January 2012. pp. 54–. ISBN 978-1-60917-326-5. https://books.google.com/books?id=70vvFrtpePoC&pg=PT54.
- ↑ 5.0 5.1 5.2 "Marketing Menopause: Science and the Public Relations of Premarin". Women, Health and Nation: Canada and the United States Since 1945. McGill-Queen's Press - MQUP. 2003. pp. 103–. ISBN 978-0-7735-2501-6. https://books.google.com/books?id=CRjtHlq1INcC&pg=PA103.
- ↑ "Estrogens used in current menopause therapies". Managing the Menopause. Cambridge University Press. 20 August 2015. pp. 118–123. ISBN 978-1-107-45182-7. https://books.google.com/books?id=l0pLCgAAQBAJ&pg=PA118.
- ↑ 7.0 7.1 "Functional role of estrogen metabolism in target cells: review and perspectives". Carcinogenesis 19 (1): 1–27. January 1998. doi:10.1093/carcin/19.1.1. PMID 9472688.
- ↑ "Metabocard for Estriol-3-glucuronide". Human Metabolome Database. http://www.hmdb.ca/metabolites/HMDB10335.
- ↑ "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens". Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Handbook of Experimental Pharmacology. 135 / 2. Springer Science & Business Media. 6 December 2012. pp. 261-322 (265). doi:10.1007/978-3-642-60107-1_15. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA265.
- ↑ "Estriol metabolism in the baboon: analysis of urinary and biliary metabolites". Steroids 22 (6): 795–817. December 1973. doi:10.1016/0039-128X(73)90054-8. PMID 4203562.
External links
 |

