Chemistry:3β-Androstenol
From HandWiki
Short description: Chemical compound
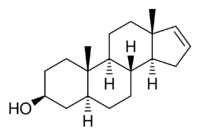
| |
| Names | |
|---|---|
| IUPAC name
5α-Androst-16-en-3β-ol
| |
| Systematic IUPAC name
(3aS,3bR,5aS,7S,9aS,9bS,11aR)-9a,11a-Dimethyl-3a,3b,4,5,5a,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-3H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H30O | |
| Molar mass | 274.448 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3β-Androstenol, also known as 5α-androst-16-en-3β-ol, is a naturally occurring mammalian pheromone known to be present in humans and pigs.[1][2][3] It is thought to play a role in axillary odor.[3] It is produced from androstenone via the enzyme 3β-hydroxysteroid dehydrogenase.[1] Unlike its C3α epimer 3α-androstenol, 3β-androstenol shows no potentiation of the GABAA receptor or anticonvulsant activity.[4]
See also
References
- ↑ 1.0 1.1 "Body malodours and their topical treatment agents". International Journal of Cosmetic Science 33 (4): 298–311. 2011. doi:10.1111/j.1468-2494.2011.00649.x. PMID 21401651.
- ↑ Richard L. Doty (27 January 2010). The Great Pheromone Myth. JHU Press. pp. 139–. ISBN 978-0-8018-9347-6. https://books.google.com/books?id=oZmk-XWeAn8C&pg=PA139.
- ↑ 3.0 3.1 "Development of a candidate reference method for the simultaneous quantitation of the boar taint compounds androstenone, 3α-androstenol, 3β-androstenol, skatole, and indole in pig fat by means of stable isotope dilution analysis-headspace solid-phase microextraction-gas chromatography/mass spectrometry". Anal. Chem. 83 (17): 6785–91. 2011. doi:10.1021/ac201465q. PMID 21800819.
- ↑ "Metabolism of the 16-androstene steroids in primary cultured porcine hepatocytes". J. Steroid Biochem. Mol. Biol. 96 (1): 79–87. 2005. doi:10.1016/j.jsbmb.2005.01.030. PMID 15896952.
 |
