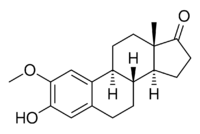
Chemistry:2-Methoxyestrone
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3-Hydroxy-2-methoxyestra-1,3,5(10)-trien-17-one
| |
| Systematic IUPAC name
(3aS,3bR,9bS,11aS)-7-Hydroxy-8-methoxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-1-one | |
| Other names
2-ME1; 2-MeOE1; 2-MeO-E1; 2-Hydroxyestrone 2-methyl ether; 2-Methoxyestra-1,3,5(10)-trien-3-ol-17-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H24O3 | |
| Molar mass | 300.398 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Methoxyestrone (2-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 2-hydroxyestrone.[1][2][3] Unlike estrone but similarly to 2-hydroxyestrone and 2-methoxyestradiol, 2-methoxyestrone has very low affinity for the estrogen receptor and lacks significant estrogenic activity.[4]
See also
References
- ↑ "Human Metabolome Database: Showing metabocard for 2-Methoxyestrone (HMDB0000010)". http://www.hmdb.ca/metabolites/hmdb00010.
- ↑ Hemnes, Anna R. (16 December 2015). Gender, Sex Hormones and Respiratory Disease: A Comprehensive Guide. Humana Press. pp. 32–. ISBN 978-3-319-23998-9. https://books.google.com/books?id=4So3CwAAQBAJ&pg=PA32.
- ↑ Lauritzen, Christian; Studd, John W. W. (22 June 2005). Current Management of the Menopause. CRC Press. pp. 378–379. ISBN 978-0-203-48612-2. https://books.google.com/books?id=WD7S7677xUUC&pg=PA378.
- ↑ "Comparison of pharmacokinetics of a conjugated equine estrogen preparation (premarin) and a synthetic mixture of estrogens (C.E.S.) in postmenopausal women". Journal of the Society for Gynecologic Investigation 7 (3): 175–83. 2000. doi:10.1016/s1071-5576(00)00049-6. PMID 10865186.
External links
 |
