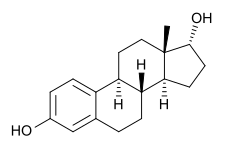
Chemistry:17α-Estradiol
| |
| Names | |
|---|---|
| IUPAC name
Estra-1,3,5(10)-triene-3,17α-diol
| |
| Systematic IUPAC name
(1R,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,7-diol | |
| Other names
17α-E2; Alpha-Estradiol; Alfatradiol; 17-Epiestradiol; β-Estradiol (obsolete, misleading)[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H24O2 | |
| Molar mass | 272.388 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
17α-Estradiol (also known as 17α-E2, 17-epiestradiol, alfatradiol, or estra-1,3,5(10)-triene-3,17α-diol) is a minor and weak endogenous steroidal estrogen that is related to 17β-estradiol (better known simply as estradiol).[2] It is the C17 epimer of estradiol.[2] It has approximately 100-fold lower estrogenic potency than 17β-estradiol.[3] The compound shows preferential affinity for the ERα over the ERβ.[2][4] Although 17α-estradiol is far weaker than 17β-estradiol as an agonist of the nuclear estrogen receptors, it has been found to bind to and activate the brain-expressed ER-X with a greater potency than that of 17β-estradiol, suggesting that it may be the predominant endogenous ligand for the receptor.[5]
Biosynthesis
17α-Estradiol is produced from epitestosterone by aromatase at locations not fully characterized (known to include the brain). Where and how epitestosterone is made is not fully understood. Conversion between 17α-estradiol and estrone seems to occur, but the enzymes remain unidentified.[5]
Occurrence
17α-E2 is found in mice brain, regardless of age and sex, at concentrations much higher than 17β-E2. Gonadectomized and/or adrenalectomized mice continue to have high brain levels of 17α-E2.[5]
17α-E2 poorly binds α-fetoprotein, unlike 17β-E2.[5]
17α-E2 is excreted in urine. It was initially discovered in pregnant mare urine (see conjugated estrogens).[5] In a 2022 study, all six tested human urine samples contained detectable amounts of 17α-E2.[6]
Function
As mentioned before, 17α-estradiol binds to ERα and ERβ with moderate affinity but very low activity. It binds to the brain-localized ER-X with significant activity and may play a neuroprotective role.[5]
In the uterus, 17α-estradiol causes smooth muscle relaxation via a nongenomic pathway, similarly to 17β-estradiol; the effect is weaker with no antagonization. It antagonizes the hypertrophic response of 17β-estradiol, probably by acting as an antiestrogen by virtue of its very low activity.[7]
Aging
Supplementation with 17α-Estradiol increases the median lifespan of male mice by 19%, while not affecting female lifespan. This treatment does not lead to feminization of male mice.[8] 17α-Estradiol furthermore alleviates age-related metabolic and inflammatory dysfunction[9] and improves glucose tolerance[10] in male mice. The exact reason for this sex-specific increase in lifespan is unknown, however, the effect on male lifespan is gone in castrated mice, suggesting that the metabolic response to 17α-Estradiol requires the presence of male gonadal hormones.[11] Whether these results are translatable to humans is currently unknown.
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA897.
- ↑ 2.0 2.1 2.2 "Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding". Endocrinology 147 (9): 4132–50. 2006. doi:10.1210/en.2006-0113. PMID 16728493.
- ↑ Ralph M. Trüeb; Won-Soo Lee (13 February 2014). Male Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 93–. ISBN 978-3-319-03233-7. https://books.google.com/books?id=0ue5BAAAQBAJ&pg=PA93.
- ↑ "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology 138 (3): 863–70. 1997. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "17alpha-estradiol: a brain-active estrogen?". Endocrinology 146 (9): 3843–50. 2005. doi:10.1210/en.2004-1616. PMID 15947006.
- ↑ Tang, Z; Liu, ZH; Wang, H; Dang, Z (10 July 2022). "17α-Estradiol, an ignored endogenous natural estrogen in human: Updated estrogen metabolism pathways and its environmental risk analysis.". The Science of the Total Environment 829: 154693. doi:10.1016/j.scitotenv.2022.154693. PMID 35318059. Bibcode: 2022ScTEn.829o4693T.
- ↑ Perusquía, M; Navarrete, E (21 July 2005). "Evidence that 17alpha-estradiol is biologically active in the uterine tissue: antiuterotonic and antiuterotrophic action.". Reproductive Biology and Endocrinology 3: 30. doi:10.1186/1477-7827-3-30. PMID 16042770.
- ↑ Strong, Randy; Miller, Richard A.; Antebi, Adam; Astle, Clinton M.; Bogue, Molly; Denzel, Martin S.; Fernandez, Elizabeth; Flurkey, Kevin et al. (October 2016). "Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer" (in en). Aging Cell 15 (5): 872–884. doi:10.1111/acel.12496. ISSN 1474-9718. PMID 27312235.
- ↑ Stout, Michael B.; Steyn, Frederik J.; Jurczak, Michael J.; Camporez, Joao-Paulo G.; Zhu, Yi; Hawse, John R.; Jurk, Diana; Palmer, Allyson K. et al. (2016-01-24). "17α-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization". The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 72 (1): 3–15. doi:10.1093/gerona/glv309. ISSN 1079-5006. PMID 26809497. PMC 5155656. https://doi.org/10.1093/gerona/glv309.
- ↑ Garratt, Michael; Bower, Brian; Garcia, Gonzalo G.; Miller, Richard A. (December 2017). "Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC 2 signaling" (in en). Aging Cell 16 (6): 1256–1266. doi:10.1111/acel.12656. ISSN 1474-9718. PMID 28834262.
- ↑ Garratt, Michael; Lagerborg, Kim A.; Tsai, Yi-Miau; Galecki, Andrzej; Jain, Mohit; Miller, Richard A. (August 2018). "Male lifespan extension with 17-α estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice" (in en). Aging Cell 17 (4): e12786. doi:10.1111/acel.12786. PMID 29806096.
 |
