Chemistry:Tetraiodine nonoxide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| I4O9 | |
| Molar mass | 651.609 g·mol−1 |
| Appearance | light yellow solid[1] |
| Melting point | 75 °C (decomposes)[1] |
| reacts | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
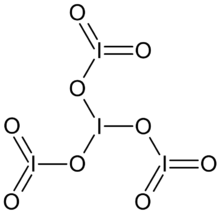
Tetraiodine nonoxide is an iodine oxide with the chemical formula I4O9.
Preparation
Tetraiodine nonoxide can be produced by reacting ozone and iodine in carbon tetrachloride at −78 °C:[2][3]
- [math]\ce{ 2I2 + 9O3 -> I4O9 + 9O2 }[/math]
It can also be produced by heating iodic acid and phosphoric acid together:[4]
- [math]\ce{ 8 HIO3 -> 2 I4O9 + 4 H2O + O2 }[/math]
Properties
Tetraiodine nonoxide is a light yellow solid that can easily hydrolyze. It decomposes above 75 °C:[2]
- [math]\ce{ 4 I4O9 -> 6 I2O5 + 2 I2 + 3 O2 }[/math]
Like diiodine tetroxide, tetraiodine nonoxide contains both I(III) and I(V), and disproportionate to iodate and iodide under alkaline conditions:[2]
- [math]\ce{ 3 I4O9 + 12 OH- -> I- + 11 IO3- + 6 H2O }[/math].
It reacts with water to form iodic acid and iodine:[3]
- [math]\ce{ 4I4O9 + 9H2O -> 18HIO3 + I2 }[/math]
References
- ↑ 1.0 1.1 Perry, Dale L. (2011). Handbook of Inorganic Compounds. Boca Raton, FL. p. 500. ISBN 978-1-4398-1462-8. OCLC 759865801.
- ↑ 2.0 2.1 2.2 Holleman, A. F.; Nils, Wiberg; Wiberg, Egon (2019) (in de). Lehrbuch der anorganischen Chemie. Berlin. p. 443. ISBN 978-3-11-083817-6. OCLC 1102802853.
- ↑ 3.0 3.1 Garg, Ragni; Singh, Randhir (2015). Inorganic Chemistry. New Delhi: McGraw-Hill Education. ISBN 978-1-259-06285-8. OCLC 965462199.
- ↑ Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry V1.. Burlington: Elsevier Science. p. 331. ISBN 978-0-323-16127-5. OCLC 843200092.
 |

