Engineering:Measuring instrument


A measuring instrument is a device to measure a physical quantity. In the physical sciences, quality assurance, and engineering, measurement is the activity of obtaining and comparing physical quantities of real-world objects and events. Established standard objects and events are used as units, and the process of measurement gives a number relating the item under study and the referenced unit of measurement. Measuring instruments, and formal test methods which define the instrument's use, are the means by which these relations of numbers are obtained. All measuring instruments are subject to varying degrees of instrument error and measurement uncertainty. These instruments may range from simple objects such as rulers and stopwatches to electron microscopes and particle accelerators. Virtual instrumentation is widely used in the development of modern measuring instruments.
Time

In the past, a common time measuring instrument was the sundial. Today, the usual measuring instruments for time are clocks and watches. For highly accurate measurement of time an atomic clock is used. Stopwatches are also used to measure time in some sports.
Energy
Energy is measured by an energy meter. Examples of energy meters include:
Electricity meter
An electricity meter measures energy directly in kilowatt-hours.
Gas meter
A gas meter measures energy indirectly by recording the volume of gas used. This figure can then be converted to a measure of energy by multiplying it by the calorific value of the gas.
Power (flux of energy)
A physical system that exchanges energy may be described by the amount of energy exchanged per time-interval, also called power or flux of energy.
- (see any measurement device for power below)
For the ranges of power-values see: Orders of magnitude (power).
Action
Action describes energy summed up over the time a process lasts (time integral over energy). Its dimension is the same as that of an angular momentum.
- A phototube provides a voltage measurement which permits the calculation of the quantized action (Planck constant) of light. (See also Photoelectric effect.)
Geometry
Dimensions (size)
Length (distance)
For the ranges of length-values see: Orders of magnitude (length)
Area
For the ranges of area-values see: Orders of magnitude (area)
Volume
- Buoyant weight (solids)
- Eudiometer, pneumatic trough (gases)
- Flow measurement devices (liquids)
- Graduated cylinder (liquids)
- Measuring cup (grained solids, liquids)
- Overflow trough (solids)
- Pipette (liquids)
If the mass density of a solid is known, weighing allows to calculate the volume.
For the ranges of volume-values see: Orders of magnitude (volume)
Angle
- Circumferentor
- Cross staff
- Goniometer
- Graphometer
- Inclinometer
- Mural instrument
- Protractor
- Quadrant
- Reflecting instruments
- Theodolite and total station
Orientation in three-dimensional space
See also the section about navigation below.
Level
Direction
Mechanics
This includes basic quantities found in classical- and continuum mechanics; but strives to exclude temperature-related questions or quantities.
Mass- or volume flow measurement
- Gas meter
- Mass flow meter
- Metering pump
- Water meter
Speed or velocity (flux of length)
- Airspeed indicator
- LIDAR speed gun
- Radar speed gun, a Doppler radar device, using the Doppler effect for indirect measurement of velocity.
- Speedometer
- Tachometer (speed of rotation)
- Tachymeter
- Variometer (rate of climb or descent)
- Velocimetry (measurement of fluid velocity)
For the ranges of speed-values see: Orders of magnitude (speed)
Acceleration
Mass

- Balance
- Check weigher measures precise weight of items in a conveyor line, rejecting underweight or overweight objects.
- Inertial balance
- Katharometer
- Mass spectrometers measure the mass-to-charge ratio, not the mass, of ionised particles.
- Weighing scale
For the ranges of mass-values see: Orders of magnitude (mass)
Linear momentum
Force (flux of linear momentum)
- Force gauge
- Spring scale
- Strain gauge
- Torsion balance
- Tribometer
Pressure (flux density of linear momentum)
- Anemometer (measures wind speed)
- Barometer used to measure the atmospheric pressure.
- Manometer (see Pressure measurement and Pressure sensor)
- Pitot tube (measures airspeed)
- Tire-pressure gauge in industry and mobility
For the ranges of pressure-values see: Orders of magnitude (pressure)
Angular velocity or rotations per time unit
For the value-ranges of angular velocity see: Orders of magnitude (angular velocity)
For the ranges of frequency see: Orders of magnitude (frequency)
Torque
- Dynamometer
- Prony brake
- Torque wrench
Energy carried by mechanical quantities, mechanical work
- Ballistic pendulum, indirectly by calculation and or gauging
Electricity, electronics, and electrical engineering
Considerations related to electric charge dominate electricity and electronics. Electrical charges interact via a field. That field is called electric field.If the charge doesn't move. If the charge moves, thus realizing an electric current, especially in an electrically neutral conductor, that field is called magnetic. Electricity can be given a quality — a potential. And electricity has a substance-like property, the electric charge. Energy (or power) in elementary electrodynamics is calculated by multiplying the potential by the amount of charge (or current) found at that potential: potential times charge (or current). (See Classical electromagnetism and Covariant formulation of classical electromagnetism)

Electric charge
- Electrometer is often used to reconfirm the phenomenon of contact electricity leading to triboelectric sequences.
- Torsion balance used by Coulomb to establish a relation between charges and force, see above.
For the ranges of charge values see: Orders of magnitude (charge)
Electric current (current of charge)
- Ammeter
- Clamp meter
- d'Arsonval galvanometer
- Galvanometer
Voltage (electric potential difference)
- Oscilloscope allows quantifying time-dependent voltages
- Voltmeter
Electric resistance, electrical conductance, and electrical conductivity
- Ohmmeter
- Time-domain reflectometer characterizes and locates faults in metallic cables by runtime measurements of electric signals.
- Wheatstone bridge
Electric capacitance
Electric inductance
- Inductance meter
Energy carried by electricity or electric energy
Power carried by electricity (current of energy)
Electric field (negative gradient of electric potential, voltage per length)
Magnetic field
See also the relevant section in the article about the magnetic field.
For the ranges of magnetic field see: Orders of magnitude (magnetic field)
Combination instruments
- Multimeter, combines the functions of ammeter, voltmeter, and ohmmeter as a minimum.
- LCR meter, combines the functions of ohmmeter, capacitance meter, and inductance meter. Also called component bridge due to the bridge circuit method of measurement.
Thermodynamics
Temperature-related considerations dominate thermodynamics. There are two distinct thermal properties: A thermal potential — the temperature. For example: A glowing coal has a different thermal quality than a non-glowing one.
And a substance-like property, — the entropy; for example: One glowing coal won't heat a pot of water, but a hundred will.
Energy in thermodynamics is calculated by multiplying the thermal potential by the amount of entropy found at that potential: temperature times entropy.
Entropy can be created by friction but not annihilated.
Amount of substance (or mole number)
- A physical quantity introduced in chemistry; usually determined indirectly. If mass and substance type of the sample are known, then atomic- or molecular masses (taken from a periodic table, masses measured by mass spectrometry) give direct access to the value of the amount of substance. (See also Molar mass.) If specific molar values are given, then the amount of substance of a given sample may be determined by measuring volume, mass, or concentration. See also the subsection below about the measurement of the boiling point.
- Gas collecting tube gases
Temperature
- Electromagnetic spectroscopy
- Galileo thermometer
- Gas thermometer principle: relation between temperature and volume or pressure of a gas (gas laws).
- Constant pressure gas thermometer
- Constant volume gas thermometer
- Liquid crystal thermometer
- Liquid thermometer principle: relation between temperature and volume of a liquid (coefficient of thermal expansion).
- Pyranometer principle: solar radiation flux density relates to surface temperature (Stefan–Boltzmann law)
- Pyrometers principle: temperature dependence of spectral intensity of light (Planck's law), i.e. the color of the light relates to the temperature of its source, range: from about −50 °C to +4000 °C, note: measurement of thermal radiation (instead of thermal conduction, or thermal convection) means: no physical contact becomes necessary in temperature measurement (pyrometry). Also note: thermal space resolution (images) found in thermography.
- Resistance thermometer principle: relation between temperature and electrical resistance of metals (platinum) (electrical resistance), range: 10 to 1,000 kelvins, application in physics and industry
- Solid thermometer principle: relation between temperature and length of a solid (coefficient of thermal expansion).
- Thermistors principle: relation between temperature and electrical resistance of ceramics or polymers, range: from about 0.01 to 2,000 kelvins (−273.14 to 1,700 °C)
- Thermocouples principle: relation between temperature and voltage of metal junctions (Seebeck effect), range: from about −200 °C to +1350 °C
- Thermometer
- Thermopile is a set of connected thermocouples
- Triple point cell used for calibrating thermometers.
Imaging technology
- Thermographic camera uses a microbolometer for detection of heat radiation.
For the ranges of temperature-values see: Orders of magnitude (temperature)
Energy carried by entropy or thermal energy
This includes thermal mass or temperature coefficient of energy, reaction energy, heat flow, ... Calorimeters are called passive if gauged to measure emerging energy carried by entropy, for example from chemical reactions. Calorimeters are called active or heated if they heat the sample, or reformulated: if they are gauged to fill the sample with a defined amount of entropy.
- Actinometer heating power of radiation.
- Constant-temperature calorimeter, phase change calorimeter for example an ice calorimeter or any other calorimeter observing a phase change or using a gauged phase change for heat measurement.
- Constant-volume calorimeter, also called bomb calorimeter
- Constant-pressure calorimeter, enthalpy-meter, or coffee cup calorimeter
- Differential Scanning Calorimeter
- Reaction calorimeter
- See also Calorimeter or Calorimetry
Entropy
Entropy is accessible indirectly by measurement of energy and temperature.
Entropy transfer
Phase change calorimeter's energy value divided by absolute temperature give the entropy exchanged. Phase changes produce no entropy and therefore offer themselves as an entropy measurement concept. Thus entropy values occur indirectly by processing energy measurements at defined temperatures, without producing entropy.
- Constant-temperature calorimeter, phase change calorimeter
- Heat flux sensor uses thermopiles (which are connected thermocouples) to determine current density or flux of entropy.
Entropy content
The given sample is cooled down to (almost) absolute zero (for example by submerging the sample in liquid helium). At absolute zero temperature any sample is assumed to contain no entropy (see Third law of thermodynamics for further information). Then the following two active calorimeter types can be used to fill the sample with entropy until the desired temperature has been reached: (see also Thermodynamic databases for pure substances)
- Constant-pressure calorimeter, enthalpy-meter, active
- Constant-temperature calorimeter, phase change calorimeter, active
Entropy production
Processes transferring energy from a non-thermal carrier to heat as a carrier do produce entropy (Example: mechanical/electrical friction, established by Count Rumford). Either the produced entropy or heat are measured (calorimetry) or the transferred energy of the non-thermal carrier may be measured.
- calorimeter
- (any device for measuring the work which will or would eventually be converted to heat and the ambient temperature)
Entropy lowering its temperature—without losing energy—produces entropy (Example: Heat conduction in an isolated rod; "thermal friction").
- calorimeter
Temperature coefficient of energy or "heat capacity"
Concerning a given sample, a proportionality factor relating temperature change and energy carried by heat. If the sample is a gas, then this coefficient depends significantly on being measured at constant volume or at constant pressure. (The terminology preference in the heading indicates that the classical use of heat bars it from having substance-like properties.)
- Constant-volume calorimeter, bomb calorimeter
- Constant-pressure calorimeter, enthalpy-meter
Specific temperature coefficient of energy or "specific heat capacity"
The temperature coefficient of energy divided by a substance-like quantity (amount of substance, mass, volume) describing the sample. Usually calculated from measurements by a division or could be measured directly using a unit amount of that sample.
For the ranges of specific heat capacities see: Orders of magnitude (specific heat capacity)
Coefficient of thermal expansion
Melting temperature (of a solid)
- Differential Scanning Calorimeter gives melting point and enthalpy of fusion.
- Kofler bench
- Thiele tube
Boiling temperature (of a liquid)
- Ebullioscope a device for measuring the boiling point of a liquid. This device is also part of a method that uses the effect of boiling point elevation for calculating the molecular mass of a solvent.
See also Thermal analysis, Heat.
More on continuum mechanics
This includes mostly instruments which measure macroscopic properties of matter: In the fields of solid-state physics; in condensed matter physics which considers solids, liquids, and in-betweens exhibiting for example viscoelastic behavior. Furthermore, fluid mechanics, where liquids, gases, plasmas, and in-betweens like supercritical fluids are studied.
Density
This refers to particle density of fluids and compact(ed) solids like crystals, in contrast to bulk density of grainy or porous solids.
- Aerometer liquids
- Dasymeter gases
- Gas collecting tube gases
- Hydrometer liquids
- Pycnometer liquids
- Resonant frequency and damping analyser (RFDA) solids
For the ranges of density-values see: Orders of magnitude (density)
Hardness of a solid
- Durometer
Shape and surface of a solid
- Holographic interferometer
- Laser produced speckle pattern analysed.
- Resonant frequency and damping analyser (RFDA)
- Tribometer
Deformation of condensed matter
- Strain gauge all below
Elasticity of a solid (elastic moduli)
- Resonant frequency and damping analyser (RFDA), using the impulse excitation technique: A small mechanical impulse causes the sample to vibrate. The vibration depends on elastic properties, density, geometry, and inner structures (lattice or fissures).
Plasticity of a solid
Tensile strength, ductility, or malleability of a solid
Granularity of a solid or of a suspension
Viscosity of a fluid
Optical activity
Surface tension of liquids
Imaging technology
- Tomograph, device and method for non-destructive analysis of multiple measurements done on a geometric object, for producing 2- or 3-dimensional images, representing the inner structure of that geometric object.
- Wind tunnel
This section and the following sections include instruments from the wide field of Category:Materials science, materials science.
More on electric properties of condensed matter, gas
Permittivity, relative static permittivity, (dielectric constant), or electric susceptibility
Such measurements also allow to access values of molecular dipoles.
Magnetic susceptibility or magnetization
For other methods see the section in the article about magnetic susceptibility.
See also Category:Electric and magnetic fields in matter
Substance potential or chemical potential or molar Gibbs energy
Phase conversions like changes of aggregate state, chemical reactions or nuclear reactions transmuting substances, from reactants into products, or diffusion through membranes have an overall energy balance. Especially at constant pressure and constant temperature, molar energy balances define the notion of a substance potential or chemical potential or molar Gibbs energy, which gives the energetic information about whether the process is possible or not - in a closed system.
Energy balances that include entropy consist of two parts: A balance that accounts for the changed entropy content of the substances, and another one that accounts for the energy freed or taken by that reaction itself, the Gibbs energy change. The sum of reaction energy and energy associated to the change of entropy content is also called enthalpy. Often the whole enthalpy is carried by entropy and thus measurable calorimetrically.
For standard conditions in chemical reactions either molar entropy content and molar Gibbs energy with respect to some chosen zero point are tabulated. Or molar entropy content and molar enthalpy with respect to some chosen zero are tabulated. (See Standard enthalpy change of formation and Standard molar entropy)
The substance potential of a redox reaction is usually determined electrochemically current-free using reversible cells.
- Redox electrode
Other values may be determined indirectly by calorimetry. Also by analyzing phase-diagrams.
Sub-microstructural properties of condensed matter, gas
- Infrared spectroscopy
- Neutron detector
- Radio frequency spectrometers for nuclear magnetic resonance and electron paramagnetic resonance
- Raman spectroscopy
Crystal structure
- An X-ray tube, a sample scattering the X-rays and a photographic plate to detect them. This constellation forms the scattering instrument used by X-ray crystallography for investigating crystal structures of samples. Amorphous solids lack a distinct pattern and are identifiable thereby.
Imaging technology, microscope
- Electron microscope
- Scanning electron microscope
- Transmission electron microscope
- Optical microscope uses reflectiveness or refractiveness of light to produce an image.
- Scanning acoustic microscope
- Scanning probe microscope
- Atomic force microscope (AFM)
- Scanning tunneling microscope (STM)
- Focus variation
- X-ray microscope
(See also Spectroscopy and List of materials analysis methods.)
Rays ("waves" and "particles")
Sound, compression waves in matter
Microphones in general, sometimes their sensitivity is increased by the reflection- and concentration principle realized in acoustic mirrors.
Sound pressure
- Microphone or hydrophone properly gauged
- Shock tube
- Sound level meter
Light and radiation without a rest mass, non-ionizing
- Antenna (radio)
- Bolometer measuring the energy of incident electromagnetic radiation.
- Camera
- EMF meter
- Interferometer used in the wide field of interferometry
- Microwave power meter
- Optical power meter
- Photographic plate
- Photomultiplier
- Phototube
- Radio telescope
- Spectrometer
- T-ray detectors
(for lux meter, see the section about human senses and human body)
See also Category:Optical devices
Photon polarization
Pressure (current density of linear momentum)
Radiant flux
The measure of the total power of light emitted.
- Integrating sphere for measuring the total radiant flux of a light source
Radiation with a rest mass, particle radiation
Cathode rays
- Crookes tube
- Cathode-ray tube, a phosphor-coated anode
Atom polarization and electron polarization
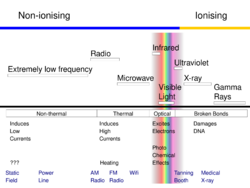
Ionizing radiation
Ionizing radiation includes rays of "particles" as well as rays of "waves". Especially X-rays and gamma rays transfer enough energy in non-thermal, (single-) collision processes to separate electron(s) from an atom.
Particle and ray flux
- Bubble chamber
- Cloud chamber
- Dosimeter, a technical device realizes different working principles.
- Geiger counter
- Ionisation chamber
- Microchannel plate detector
- Photographic plate
- Photostimulable phosphor plate
- Proportional counter
- Scintillation counter, Lucas cell
- Semiconductor detector
Identification and content
This could include chemical substances, rays of any kind, elementary particles, and quasiparticles. Many measurement devices outside this section may be used or at least become part of an identification process. For identification and content concerning chemical substances, see also Analytical chemistry, List of chemical analysis methods, and List of materials analysis methods.
Substance content in mixtures, substance identification
- Carbon dioxide sensor
- chromatographic device, gas chromatograph separates mixtures of substances. Different velocities of the substance types accomplish the separation.
- Colorimeter absorbance, and thus concentration
- Gas detector
- Gas detector in combination with mass spectrometer,
- mass spectrometer identifies the chemical composition of a sample on the basis of the mass-to-charge ratio of charged particles.
- Nephelometer or turbidimeter
- Oxygen sensor (= lambda sond)
- Refractometer, indirectly by determining the refractive index of a substance.
- Smoke detector
- Ultracentrifuge, separates mixtures of substances. In a force field of a centrifuge, substances of different densities separate.
pH: Concentration of protons in a solution
Humidity
- Hygrometer the density of water in air
- Lysimeter the balance of water in soil
Human senses and human body
Sight
Brightness: photometry
Photometry is the measurement of light in terms of its perceived brightness to the human eye. Photometric quantities derive from analogous radiometric quantities by weighting the contribution of each wavelength by a luminosity function that models the eye's spectral sensitivity. For the ranges of possible values, see the orders of magnitude in: illuminance, luminance, and luminous flux.
- Photometers of various kinds:
- Lux meter for measuring illuminance, i.e. incident luminous flux per unit area
- Luminance meter for measuring luminance, i.e. luminous flux per unit area and unit solid angle
- Light meter, an instrument used to set photographic exposures. It can be either a lux meter (incident-light meter) or a luminance meter (reflected-light meter), and is calibrated in photographic units.
- Integrating sphere for collecting the total luminous flux of a light source, which can then be measured by a photometer
- Densitometer for measuring the degree to which a photographic material reflects or transmits light
Color: colorimetry
- Tristimulus colorimeter for quantifying colors and calibrating an imaging workflow
Radar brightness: radiometry
Synthetic Aperture Radar (SAR) instruments measure radar brightness, Radar Cross Section (RCS), which is a function of the reflectivity and moisture of imaged objects at wavelengths which are too long to be perceived by the human eye. Black pixels mean no reflectivity (e.g. water surfaces), white pixels mean high reflectivity (e.g. urban areas). Colored pixels can be obtained by combining three gray-scaled images which usually interpret the polarization of electromagnetic waves. The combination R-G-B = HH-HV-VV combines radar images of waves sent and received horizontally (HH), sent horizontally and received vertically (HV) and sent and received vertically (VV). The calibration of such instruments is done by imaging objects (calibration targets) whose radar brightness is known.
Hearing
Loudness in phon
- Headphone, loudspeaker, sound pressure gauge, for measuring an equal-loudness contour of a human ear.
- Sound level meter calibrated to an equal-loudness contour of the human auditory system behind the human ear.
Smell
- Olfactometer, see also Olfaction.
Temperature (sense and body)
Body temperature or core temperature
- Medical thermometer, see also infrared thermometer
Circulatory system (mainly heart and blood vessels for distributing substances fast)
Blood-related parameters are listed in a blood test.
- Electrocardiograph records the electrical activity of the heart
- Glucose meter for obtaining the status of blood sugar.
Respiratory system (lung and airways controlling the breathing process)
- Spirometer
Concentration or partial pressure of carbon dioxide in the respiratory gases
Nervous system (nerves transmitting and processing information electrically)
- Electroencephalograph records the electrical activity of the brain
Musculoskeletal system (muscles and bones for movement)
power, work of muscles
metabolic system
- Body fat meter
Medical imaging
- Computed tomography
- Magnetic resonance imaging
- Medical ultrasonography
- Radiology
- Tomograph, device and method for non-destructive analysis of multiple measurements done on a geometric object, for producing 2- or 3-dimensional images, representing the inner structure of that geometric object.
See also: Category:Physiological instruments and Category:Medical testing equipment.
Meteorology
See also Category:Meteorological instrumentation and equipment.
See also Category:Navigational equipment and Category:Navigation. See also Surveying instruments.
Astronomy
Military
Some instruments, such as telescopes and sea navigation instruments, have had military applications for many centuries. However, the role of instruments in military affairs rose exponentially with the development of technology via applied science, which began in the mid-19th century and has continued through the present day. Military instruments as a class draw on most of the categories of instrument described throughout this article, such as navigation, astronomy, optics, and imaging, and the kinetics of moving objects. Common abstract themes that unite military instruments are seeing into the distance, seeing in the dark, knowing an object's geographic location, and knowing and controlling a moving object's path and destination. Special features of these instruments may include ease of use, speed, reliability, and accuracy.
Uncategorized, specialized, or generalized application
- Actograph measures and records animal activity within an experimental chamber.
- Densitometer measures light transmission through processed photographic film or transparent material or light reflection from a reflective material.
- Force platform measures ground reaction force.
- Gauge (engineering) A highly precise measurement instrument, also usable to calibrate other instruments of the same kind. Often found in conjunction with defining or applying technical standards.
- Gradiometer any device that measures spatial variations of a physical quantity. For example, as done in gravity gradiometry.
- Parking meter measures time a vehicle is parked at a particular spot, usually with a fee.
- Postage meter measures postage used from a prepaid account.
- S meter measures the signal strength processed by a communications receiver.
- Sensor, hypernym for devices that measure with little interaction, typically used in technical applications.
- Spectroscope is an important tool used by physicists.
- SWR meter check the quality of the match between the antenna and the transmission line.
- Universal measuring machine measures geometric locations for inspecting tolerances.
Alphabetical listing
| Instrument | Quantity measured |
|---|---|
| alcoholmeter | alcoholic strength of liquid |
| altimeter | altitude |
| ammeter | electric current |
| anemometer | windspeed |
| astrolabe | latitude and altitude of celestial bodies |
| audiometer | hearing |
| barkometer | tanning liquors used in tanning leather |
| barometer | air pressure |
| bettsometer | integrity of fabric coverings on aircraft |
| bevameter | mechanical properties of soil |
| bolometer | electromagnetic radiation |
| Brannock Device | measuring shoe size |
| breathalyzer | breath alcohol content |
| caliper | length |
| calorimeter | heat of chemical reactions |
| cathetometer | vertical distances |
| ceilometer | height of a cloud base |
| chronometer or clock | time |
| clap-o-meter | volume of applause |
| compass | direction of North |
| Coulombmeter | electrostatic charge of a material |
| colorimeter | color |
| creepmeter | slow surface displacement of an active geologic fault in the earth |
| corrator | corrosion rate |
| declinometer | magnetic declination |
| densimeter | specific gravity of liquids |
| densitometer | degree of darkness in photographic or semitransparent material |
| diffractometer | structure of crystals |
| dilatometer | volume changes caused by a physical or chemical process |
| disdrometer | size, speed, and velocity of raindrops |
| dosimeter | exposure to hazards, especially radiation; radiation of item |
| drumometer | amount of drum strokes over time |
| dumpy level | horizontal levels, polar angle |
| dynamometer | force, torque, or power |
| electricity meter | electrical energy used |
| electrometer | electric charge |
| electronic tuner | pitch of musical notes |
| ellipsometer | refractive index, dielectric function, thickness of thin films |
| eudiometer | change in volume of a gas mixture following combustion |
| evaporimeter | rate of evaporation |
| fathometer | ocean depth |
| feeler gauge | gap widths |
| forward looking infrared (FLIR) | detects infrared energy (heat)converts it into an electronic signal, which is then processed to produce a thermal image on a video monitor and perform temperature calculations. |
| framing square | right angles in construction |
| frequency counter | frequency of alternating current |
| fuel gauge | fuel levels |
| galvanometer | electricity |
| gas pycnometer | volume and density of solids |
| geiger counter | ionizing radiation (alpha, beta, gamma, etc.) |
| glucometer | blood glucose (diabetes) |
| graphometer | angle |
| heliometer | variation of the sun's diameter |
| hourmeter | elapsed machine hours |
| hydrometer | specific gravity of liquids (density of liquids) |
| hygrometer | humidity |
| inclinometer | angle of a slope |
| inkometer | ink |
| interferometer | wave interference |
| infrared thermometer | heat radiated |
| katharometer | composition of gases |
| lactometer | specific gravity of milk |
| light meter | light (in photography) |
| linear position transducer | speed of movement |
| load cell | measurement of force |
| lux meter | intensity of light |
| magnetometer | strength of magnetic fields |
| manometer | pressure of gas |
| mass flow meter | mass flow rate of a fluid travelling through a tube |
| mass spectrometer | masses of ions, used to identify chemical substances through their mass spectra |
| measuring cup | liquid and dry goods |
| measuring cylinder | volume |
| measuring spoon | a spoon used to measure an amount of an ingredient, either liquid or dry |
| megger | electrical insulation |
| mercury barometer | Atmospheric pressure |
| micrometer | small distances |
| multimeter | electrical potential, resistance, and current |
| nephoscope | to measure the speed and direction of clouds |
| nephelometer | particle in a liquid |
| odometer | distance travelled |
| ohmmeter | electrical resistance |
| opisometer | lengths of arbitrary curved lines |
| orchidometer | testicle size in male humans |
| oscilloscope | oscillations |
| osmometer | osmotic strength of a solution, colloid, or compound matter of an object |
| parking meter | collects moneys for vehicle parking rights in a zone for a limited time |
| pedometer | steps |
| pH meter | pH (chemical acidity/basicity of a solution) |
| photometer | illuminance or irradiance |
| planometer | area |
| polarimeter | rotation of polarized light |
| potentiometer | voltage (term is also used to refer to a variable resistor) |
| profilometer | surface roughness |
| protractor | angle |
| psychrometer | humidity |
| pycnometer | fluid density |
| pyranometer | solar radiation |
| pyrheliometer | direct solar insolation |
| pyrometer | high temperatures |
| quadrat | percentage cover of a certain species |
| quartz crystal microbalance | thickness of deposited thin films |
| rain gauge | measuring of rain |
| radiometer | radiant flux of electromagnetic radiation |
| refractometer | index of refraction |
| rheometer | response to applied forces |
| rotameter | pressure of a liquid or gas in a closed tube |
| ruler | for measuring length |
| saccharometer | amount of sugar in a solution |
| seismometer | seismic waves (for example, earthquakes) |
| sextant | location on earth's surface (used in naval navigation) |
| spectrometer | properties of light |
| spectrophotometer | intensity of light as a function of wavelength |
| speedometer | speed, velocity of a vehicle |
| spirometer | the lung capacity |
| spherometer | radius of a sphere |
| sphygmomanometer | blood pressure |
| stadimeter | object range |
| strainmeter | seismic strain |
| SWR meter | standing wave ratio |
| Synthetic Aperture Radar | reflectivity and moisture |
| tacheometer | distance |
| tachometer | revolutions per minute, rate of blood flow, speed of aeroplanes |
| taximeter | distance travelled, displacement |
| tensiometer | surface tension of a liquid |
| theodolite | angle, in the horizontal and vertical planes |
| thermometer | temperature |
| tiltmeter | minor changes to the Earth |
| tintometer | colour |
| universal measuring machine | geometric locations |
| vacuum gauge | very low pressure |
| viscometer | viscosity of a fluid |
| voltmeter | electric potential, voltage |
| VU meter | volume unit |
| wattmeter | electrical power |
| weighing scale | weight |
| wind vane | wind direction |
| zymometer | fermentation |
See also
- Category:Instrument-making corporations
- Data logger measuring devices
- History of measurement
- History of weights and measures
- Instrumentation
- List of measuring devices
- List of physical quantities
- List of sensors
- Metrology
- Pocket comparator
- Sensor or detector
- Timeline of temperature and pressure measurement technology
Notes
The alternate spelling "-metre" is never used when referring to a measuring device.
References
External links