Chemistry:Rhenium(VII) oxide
| |

| |
| Names | |
|---|---|
| Other names
Rhenium heptoxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Re2O7 | |
| Molar mass | 484.40298 g/mol |
| Appearance | yellow crystalline powder |
| Density | 6.103 g/cm3, solid |
| Melting point | 360 °C (680 °F; 633 K) |
| Boiling point | sublimes |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| Related compounds | |
Related compounds
|
Manganese(VII) oxide; technetium(VII) oxide; perrhenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.[2]
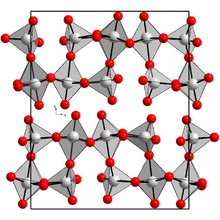
Structure
Solid Re2O7 consists of alternating octahedral and tetrahedral Re centres. Upon heating, the polymer cracks to give molecular (nonpolymeric) Re2O7. This molecular species closely resembles manganese heptoxide, consisting of a pair of ReO4 tetrahedra that share a vertex, i.e., O3Re–O–ReO3.[3]
Synthesis and reactions
Rhenium(VII) oxide is formed when metallic rhenium or its oxides or sulfides are oxidized at 500–700 °C (900–1,300 °F) in air.[4]
Re2O7 dissolves in water to give perrhenic acid.
Heating Re2O7 gives rhenium dioxide, a reaction signalled by the appearance of the dark blue coloration:[5]
- 2Re2O7 → 4ReO2 + 3O2
Using tetramethyltin, it converts to methylrhenium trioxide ("MTO"), a catalyst for oxidations:[6]
- Re2O7 + 2Sn(CH3)4 → CH3ReO3 + (CH3)3SnOReO3
In a related reaction, it reacts with hexamethyldisiloxane to give the siloxide:[4]
- Re2O7 + 2O(Si(CH3)3)2 → 2(CH3)3SiOReO3
Uses
Hydrogenation catalyst
Rhenium(VII) oxide finds some use in organic synthesis as a catalyst for ethenolysis,[7] carbonyl reduction and amide reduction.[8]
References
- ↑ "Rhenium(VII) oxide" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/123106#section=Safety-and-Hazards.
- ↑ Georg Nadler, Hans (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_199.
- ↑ Wells, A.F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ 4.0 4.1 Schmidt, Max; Schmidbaur, Hubert (1967). Trimethylsilyl Perrhenate. Inorganic Syntheses. 9. pp. 149–151. doi:10.1002/9780470132401.ch40. ISBN 9780470132401.
- ↑ O. Glemser (1963). "Rhenium". in G. Brauer. Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). Academic Press. pp. 1476–1485.
- ↑ W. A. Herrmann; F. E. Kuhn (1997). "Organorhenium Oxides". Acc. Chem. Res. 30 (4): 169–180. doi:10.1021/ar9601398.
- ↑ Lionel Delaude; Alfred F. Noels. "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley.
- ↑ Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 42-43, 182, 389-390, & 408. ISBN 9780471396987. https://books.google.com/books?id=RjZRAAAAMAAJ&q=0471396982.
 |

