Chemistry:Caesium superoxide
From HandWiki
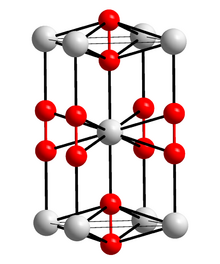 Caesium cations, Cs+ Superoxide anions, O− 2 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| CsO2 | |
| Molar mass | 164.903 g·mol−1 |
| Appearance | Yellow to orange solid [1] |
| Density | 3.77 g/cm3[1] |
| Melting point | 600 °C[2] |
| reacts | |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Caesium superoxide is a chemical compound with the chemical formula CsO
2. It consists of caesium cations Cs+
and superoxide anions O−
2. It is an orange solid.
Preparation
Burning caesium in excess oxygen will produce caesium superoxide.[2]
- Cs + O
2 → CsO
2
Properties
Caesium superoxide's crystal structure is same as calcium carbide. It contains direct oxygen-oxygen bonding.[2]
It reacts with water to form hydrogen peroxide and caesium hydroxide.[2]
- 2 CsO
2 + 2 H
2O → O
2↑ + H
2O
2 + 2 CsOH
Heating to approximately 400 °C induces thermal decomposition to caesium peroxide.[3]
The standard enthalpy of formation ΔHf0 of caesium superoxide is −295 kJ/mol.[4]
Caesium superoxide reacts with ozone to form caesium ozonide.[2]
- CsO
2 + O
3 → CsO
3 + O
2
References
- ↑ 1.0 1.1 Caesiumhyperoxid bei webelements.com.
- ↑ 2.0 2.1 2.2 2.3 2.4 Holleman, Arnold (2007) (in de). Lehrbuch der anorganischen Chemie. BerlinNew York: de Gruyter. ISBN 978-3-11-017770-1. OCLC 180963521.
- ↑ Berardinelli, S. P.; Kraus, D. L. (1974-01-01). "Thermal decomposition of the higher oxides of cesium in the temperature range 320-500.deg.". Inorganic Chemistry (American Chemical Society (ACS)) 13 (1): 189–191. doi:10.1021/ic50131a037. ISSN 0020-1669.
- ↑ Holleman, Arnold (2017) (in de). Anorganische ChemienBand 1. Berlin: de Gruyter. ISBN 978-3-11-049585-0. OCLC 968134975.
 |

